Abstract
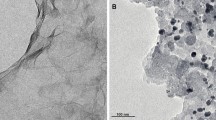
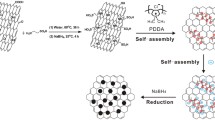
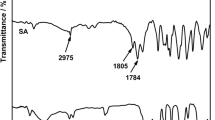
A simple and cost-effective electrochemical method synthesized platinum nanoparticles on graphene nanosheet (PtNPs@GNS) is reported, and the Pt loading of the PtNPs@GNS can be controlled by electrodeposition. The structure and element analysis of the PtNPs@GNS have been investigated by scanning electron microscopy (SEM), Raman spectrum, X-ray diffraction (XRD) and energy dispersive spectroscopy (EDS). The electrochemical measurement including electrochemical active surface area, current density, mass activity, oxidation peak potential,shows the PtNPs@GNS have more performance electrocatalytic properties for methanol oxidation reaction (MOR) compared to Vulcan XC-72 carbon (XC-72) supported PtNPs electrocatalysts. Probably, the cause which may be attributes to no aggregation of PtNPs and the well-dispersion on surface of GNS, so PtNPs@GNS show large electrochemically active surface area, highly electrocatalytic activity and stability in direct methanol fuel cells.





Similar content being viewed by others
References
M. Jafarian, M. G. Mahjani, H. Heli, F. Gobal, H. Khajehsharifi, and M. H. Hamedi (2003). Electrochim. Acta 48, 3423.
L. F. Dong, R. S. G. Raghavendar, Z. Li, M. C. Michael, and S. F. Hou (2010). Carbon 48, 781.
H. S. Liu, C. J. Song, L. Zhang, J. J. Zhang, H. J. Wang, and D. P. Wilkinson (2006). J. Power Sources 155, 95.
E. P. Lee, Z. M. Peng, W. Chen, S. W. Chen, H. Yang, and Y. N. Xia (2008). ACS Nano 2, 2167.
S. M. Choi, M. H. Seo, H. J. Kim, and W. B. Kim (2011). Carbon 49, 904.
Y. M. Li, L. H. Tang, and J. H. Li (2009). Electrochem. Common. 11, 846.
Y. J. Li, W. Gao, L. J. Ci, C. M. Wang, and P. M. Ajayan (2010). Carbon 48, 1124.
S. Liu, J. Q. Wang, J. Zeng, J. F. Ou, Z. P. Li, X. H. Liu, and S. R. Yang (2010). J. Power Sources 195, 462.
Z. Lin, L. W. Ji, and X. W. Zhang (2009). Mater. Lett. 63, 2115.
E. A. Franceschini, G. A. Planes, F. J. Williams, G. J. A. A. Soler-Illia, and H. R. Corti (2011). J. Power Sources 196, 1723.
L. Tian, Y. J. Qi, and B. B. Wang (2009). J. Colloid Interface Sci. 333, 249.
A. C. Chen, D. J. Russa, and B. Miller (2004). Langmuir 20, 9695.
K. Vinodgopal, M. Haria, D. Meisel, and P. Kamat (2004). Nano Lett. 4, 415.
H. J. Yin, H. J. Tang, D. Wang, Y. Gao, and Z. Y. Tang (2012). ACS Nano 6, 8288.
L. Cao, F. Scheiba, C. Roth, F. Schweiger, C. Cremers, U. Stimming, H. Fuess, L. Chen, W. Zhu, and X. Qiu (2006). Angew. Chem. Int. Ed. 54, 5315.
S. M. Golabi and A. Nozad (2002). J. Electroanal. Chem. 52, 161.
A. A. Mikhaylova, O. A. Khazoova, and V. S. Bagotzky (2000). J. Electroanal. Chem. 480, 225.
W. S. Li, L. P. Tian, Q. M. Huang, H. Li, H. Y. Chen, and X. P. Lian (2002). J. Power Source 104, 281.
A. G. Hubert, M. M. Nenad, and N. R. J. Philip (1995). J. Phys. Chem. 99, 8945.
H. B. Zhao, J. Yang, L. Li, H. Li, J. L. Wang, and Y. M. Zhang (2009). Int. J. Hydrogen Energy 34, 3908.
Y. G. Zhou, J. J. Chen, F. B. Wang, Z. H. Sheng, and X. H. Xia (2010). Chem. Commun. 46, 5951.
S. Bong, Y. R. Kim, I. Kim, S. Woo, S. Uhm, J. Lee, and H. Kim (2010). Electrochem. Common. 12, 129.
Y. M. Li, L. H. Tang, and J. H. Li (2009). Electrochem. Commun. 11, 846.
Y. C. Zhao, L. Zhan, J. N. Tian, S. L. Nie, and Z. Ning (2011). Electrochim. Acta 56, 1967.
S. J. Guo, S. J. Dong, and E. K. Wang (2010). ACS Nano 4, 547.
N. G. Shang, P. Pagona, P. S. Wang, and P. Ravi (2010). J. Phys. Chem. C 114, 15837.
L. Wang, C. G. Tian, H. Wang, Y. G. Ma, B. L. Wang, and H. G. Fu (2010). J. Phys. Chem. C 114, 8727.
H. C. Schniepp, J. L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud, R. Car, D. A. Saville, and I. A. Aksay (2006). J. Phys. Chem. B 110, 8535.
M. J. McAllister, J. L. LiO, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera, D. L. Milius, R. CarO, R. K. Prudhomme, and I. A. Aksay (2007). Chem. Mater. 19, 4396.
R. Kou, Y. Y. Shao, D. H. Wang, H. Mark, M. H. Engelhard, J. H. Kwak, J. Wang, C. M. Wang, Y. H. Lin, Y. Wang, A. Aksay, and J. Liu (2009). Electrochem. Commun. 11, 954.
X. Wang, L. Song, H. Y. Yang, H. D. Lu, and Y. Hu (2011). Ind. Eng. Chem. Res. 50, 5376.
S. Bourbigot, M. Bras, R. Delobel, and L. Gengembre (1997). Appl. Surf. Sci. 120, 15.
S. M. Unni, V. M. Dhavale, V. K. Pillai, and S. Kurungot (2010). J. Phys. Chem. C 114, 14654.
J. Li and X. Q. Lin (2007). J. Electrochem. Soc. 154, 1074.
J. M. Sieben, M. M. E. Duarte, and C. E. Mayer (2010). Int. J. Hydrogen Energy 35, 2018.
J. Prabhuram, T. S. Zhao, Z. K. Tang, R. Chen, and Z. X. Liang (2006). J. Phys. Chem. B 110, 5245.
G. Guo, F. Qin, D. Yang, C. Wang, H. Xu, and S. Yang (2008). Chem. Mater. 20, 2291.
W. G. Zhao, X. B. Zhou, Z. H. Xue, B. W. Wu, X. H. Liu, and X. Q. Lu (2013). J. Mater. Sci. 48, 2566.
A. C. Ferrari (2007). Solid State Commun. 143, 47.
K. Subrahmanyam, S. Manna, A. K. Pati, K. Swapan, and C. N. R. Rao (2010). Chem. Phys. Lett. 497, 70.
H. Kita, Y. Gao, T. Nakato, and H. Hattori (1994). J. Electroanal. Chem. 373, 177.
E. Herrero, K. Fanaszczuk, and A. Wiechowski (1994). J. Phys. Chem. 98, 5074.
H. M. Zhang, W. Q. Zhou, Y. K. Du, P. Yang, and C. Y. Wang (2010). Electrochem. Common. 12, 882.
Y. H. Qin, H. H. Yang, X. S. Zhang, P. Li, and C. A. Ma (2010). Int. J. Hydrogen Energy 35, 7667.
Y. Lin, X. Cui, C. Yen, and C. M. Wai (2005). J. Phys. Chem. B 109, 14410.
Y. Mu, H. Liang, J. Hu, L. Jiang, and L. Wan (2005). J. Phys. Chem. B 109, 22212.
Z. H. Wen, Q. Wang, and J. H. Li (2008). Adv. Funct. Mater. 18, 959–964.
A. Hamnett (1997). Catal. Today 38, 445.
H. Ataee-Esfahani, L. Wang, Y. Nemoto, and Y. Yamauchi (2010). Chem. Mater. 22, 6310.
S. Guo, S. Dong, and E. Wang (2010). Chem. Commun. 46, 1869.
S. Guo, J. Li, S. Dong, and E. Wang (2010). J. Phys. Chem. C 114, 15337.
Acknowledgments
The Study was supported by the National Natural Science Foundation of China (NO. 21005063, 21175108, 21165016), the Natural Science Foundation of Gansu province (No 096RJZA121) of China, and the key Laboratory of Polymer Materials of Gansu Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, W., Zhou, X., Chen, J. et al. Controllable Electrodeposition of Platinum Nanoparticles on Graphene Nanosheet for Methanol Oxidation Reaction. J Clust Sci 24, 739–748 (2013). https://doi.org/10.1007/s10876-013-0569-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0569-0