Abstract
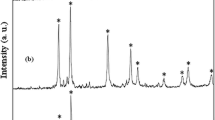
In this work, Pb(II)N,N-bis(salicylidene)-ethylenediamine; [Pb(salen)]; was applied as lead precursor to synthesis PbSe nanostructures. Besides [Pb(salen)], SeCl4 and reducing agents like N2H4·H2O have been employed for the production of PbSe nanostructures via a solvothermal route at 180 °C for 3 h in propylene glycol. The effect of preparation factors such as temperature, reaction time, and surfactant on the morphology of PbSe nanostructures was investigated. The experimental results indicated that PbSe synthesized at 150 and 210 °C was composed of agglomerated particles. On the other hand, the use of KBH4 as reducing agent led to produce PbSe with higher particle size and agglomeration. The as-prepared PbSe nanostructures were characterized by XRD, SEM, TEM, EDS, and FT-IR.






Similar content being viewed by others
References
F. W. Wise (2000). Acc. Chem. Res. 33, 773.
M. T. Harrison, S. V. Kershaw, M. G. Burt, A. L. Rogach, A. Kornowski, A. Eychmuller, and H. Weller (2000). Pure Appl. Chem. 73, 295.
R. D. Schaller and V. I. Klimov (2004). Phys. ReV. Lett. 92, 186601.
R. Ellinson, M. Beard, J. Johnson, P. Yu, O. Micic, A. Nozik, A. Shabaev, and A. Efros (2005). Nano Lett. 5, 865.
S. A. McDonald, G. Konstantatos, S. Zhang, P. W. Cyr, J. D. E. Klem, L. Levina, and E. H. Sargent (2005). Nat. Mater. 4, 138.
J. M. Pietryga, R. D. Schaller, D. Weder, M. H. Stewart, V. I. Kilmov, and J. A. Hollingsworth (2004). J. Am. Chem. Soc. 126, 11752.
D. V. Talapin and C. B. Murray (2005). Science 310, 86.
B. X. Li, Y. Xie, Y. Xu, C. Z. Wu, and Z. Q. Li (2006). J. Solid State Chem. 179, 56.
H. Tong, Y. J. Zhu, L. X. Yang, L. Li, and L. Zhang (2006). Angew. Chem. Int. Ed. 45, 7739.
Y. F. Liu, J. B. Cao, J. H. Zeng, C. Li, Y. T. Qian, and S. Y. Zhang (2003). Eur. J. Inorg. Chem. 4, 644.
W. Z. Wang, Y. Geng, Y. T. Qian, M. R. Ji, and X. M. Liu (1998). Adv. Mater. 10, 1479.
J. J. Zhu, H. Wang, S. Xu, and H. Y. Chen (2002). Langmuir 18, 3306.
A. J. Houtepen, R. Koole, D. Vanmaekelbergh, J. Meeldijk, and S. G. Hickey (2006). J. Am. Chem. Soc. 128, 6792.
W. G. Lu, J. Y. Fang, Y. Ding, and Z. L. Wang (2005). J. Phys. Chem. B 109, 19219.
X. Q. Wang, G. C. Xi, Y. K. Liu, and Y. T. Qian (2008). Cryst. Growth Des. 8, 1406.
Q. Li, Y. Ding, M. Shao, Wu Ji, G. Yu, and Y. Qi (2003). Mater. Res. Bull. 38, 539.
M. J. Bierman, Y. K. A. Lau, and S. Jin (2007). Nano Lett. 7, 2907.
J. Xu, J. Zhang, and J. Qian (2010). Mater. Lett. 64, 771.
C. Cheng, G. Xu, and H. Zhang (2009). J. Cryst. Growth. 311, 1285.
J. J. Zhu, O. Palchik, S. G. Chen, and A. Gedanken (2000). J. Phys. Chem. B. 104, 7344.
A. Sobhani, F. Davar, and M. Salavati-Niasari (2011). Appl. Surf. Sci. 257, 7982.
X. Wang, K. Li, Y. Dong, and K. Jiang (2010). Cryst. Res. Technol. 45, 94.
W.-B. Zhao, J–. J. Zhu, and H.-Y. Chen (2004). Scripta Mater. 50, 1169.
W.-K. Koh, A. C. Bartnik, F. W. Wise, and C. B. Murray (2010). J. Am. Chem. Soc. 132, 3909.
F. Mohandes, F. Davar, and M. Salavati-Niasari (2010). J. Magn. Magn. Mater. 322, 872.
M. Salavati-Niasari, N. Mir, and F. Davar (2009). J. Alloys Compd. 476, 908.
M. R. Salavati-Niasari, Estarki Loghman, and F. Davar (2008). Chem Eng. J. 145, 346.
F. Soofivand, F. Mohandes, and M. Salavati-Niasari (2012). Micro Nano Lett. 7, 283.
F. Zhang, F.-L. Bei, J.-M. Cao, and X. Wang (2008). J. Solid State Chem. 181, 143.
F. Mohandes, F. Davar, and M. Salavati-Niasari (2010). J. Phys. Chem. Solids 71, 1623.
F. Mohandes, F. Davar, M. Salavati-Niasari, and K. Saberyan (2011). Current Nanosci. 7, 260.
A. Kazemi-Babaheydari, M. Salavti-Niasari, and A. Khansari (2012). Particuology 10, 759.
R. Jenkins and R. L. Snyder Chemical Analysis: Introduction to X-ray Powder Diffractometry (John Wiley and Sons Inc., New York, 1996).
E. Esmaeili, M. Salavati-Niasari, F. Mohandes, F. Davar, and H. Seyghalkar (2011). Chem. Eng. J. 170, 278.
G. Herrera, E. Chavira, J. Jiménez-Mier, A. Ordoñez, E. Fregoso-Israel, L. Baños, E. Bucio, J. Guzmán, O. Novelo, and C. Flores (2009). J. Alloys Compd. 479, 511.
Y. Ni, B. Qiu, J. Hong, L. Zhang, and X. Wei (2008). Mater. Res. Bull. 43, 2668.
X. Ji, B. Zhang, T. M. Tritt, J. W. Kolis, and A. Kumbhar (2007). J. Electron. Mater. 36, 721.
B. Wan, C. Hu, Y. Xi, J. Xu, and X. Hec (2010). Solid State Sci. 12, 123.
Acknowledgments
Authors are grateful to council of University of Kashan for supporting this work by Grant No (159271/56), Iran National Science Foundation and IST, Jawaharlal Nehru Technological University Hyderabad and TEM section, SAIF, NEHU, Shillong, Meghalaya, India, for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salavati-Niasari, M., Shoshtari-Yeganeh, B. & Mohandes, F. Solvothermal Synthesis and Characterization of PbSe Nanostructures with the Aid of Schiff-base Ligand. J Clust Sci 24, 657–667 (2013). https://doi.org/10.1007/s10876-013-0564-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0564-5