Abstract
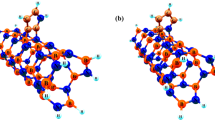
Phenol adsorption on the external surface of H-capped pristine, Ga-doped, and Pd-decorated (6,0) zigzag boron phosphide nanotubes (BPNTs) was studied by using density functional theory (DFT) calculations. The results indicate that the hydroxyl group of phenol prefers to attach to the Ga and Pd sites and thus the Ga-doped and Pd-decorated (6,0) can be used for removing phenol. The calculated adsorption energy of phenol on the Ga-doped and Pd-decorated (6,0) BPNTs are −0.724 and −420 eV, respectively and about 0.28 and 0.27 electrons are transferred from phenol to the nanotubes. In addition, the value for the fractional number of electrons transferred is negative, indicating that phenol act as an electron donor. Frontier molecular orbital theory (FMO) and structural analyses show that the high polar surface bonds and large bond lengths of the Ga-doped and Pd-decorated (6,0) BPNT surfaces increase the adsorption of phenol on the nanotube models. This study can be useful in removing phenol and development of many catalytic processes for formation of a variety of useful compounds.




Similar content being viewed by others
References
N. A. Besley and A. J. Blundy (2006). J. Phys. Chem. B 110, 1701.
G. Busca, S. Berardinelli, C. Resini, and L. Arrighi (2008). J. Hazard. Mater. 160, 265.
S. D. Chakarova and A. E. Carlsson (2004). Phys. Rev. E 69, 021907.
A. Knop and L. A. Pilato Phenolic Resins—Chemistry Applications and Performance (Springer, New York, 1985), p. 104.
H. Ihm and J. M. White (2000). J. Phys. Chem. B 104, 6202.
B. Bartlett, J. M. Valdisera, and J. N. Russell (1999). Surf. Sci. 442, 265.
J. Wallace Kirk-Othmer Encyclopedia of Chemical Technology, vol 18, 3rd ed (Wiley, New York, 2005), p. 747.
A. K. Myers and J. B. Benziger (1989). Langmuir 5, 1270.
L. Delle Site, A. Alavi, and C. F. Abrams (2008). Phys. Rev. B 67, 193406.
J. X. Zhao, B. Gao, Q. H. Cai, X. G. Wang, and X. Z. Wang (2011). Theor. Chem. Acc. 129, 85.
C. Marilena, M. Simone, and C. Ruggero (2007). Phys. Rev. B 76, 085332.
J. Karen, G. Andris, V. Tuukka, and J. P. Martti (2010). Phys. Rev. B 81, 235428.
A. Ahmadi Peyghan, M. T. Baei, M. Moghimi, and S. Hashemian (2012). Comput. Theor. Chem. doi:10.1016/j.comptc.2012.07.037.
M. Rezaei-Sameti (2012). Phys. B 407, 3717–3721.
K. Li, W. Wang, and D. Cao (2011). Sens. Actuat. B: Chem. 159, 171–177.
P. K. Chattaraj, U. Sarkar, and D. R. Roy (2006). Chem. Rev. 106, 2065.
K. K. Hazarika, N. C. Baruah, and R. C. Deka (2009). Struct. Chem. 20, 1079.
R. G. Parr, L. Szentpaly, and S. Liu (1999). J. Am. Chem. Soc. 121, 1922.
R. G. Parr and R. G. Pearson (1983). J. Am. Chem. Soc. 105, 7512.
F. Tournus and J. C. Charlier (2005). Phys. Rev. B 71, 165421.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople Gaussian 03, revision B03 (Gaussian, Pittsburgh, 2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peyghan, A.A., Baei, M.T., Moghimi, M. et al. Theoretical Study of Phenol Adsorption on Pristine, Ga-Doped, and Pd-Decorated (6,0) Zigzag Single-Walled Boron Phosphide Nanotubes. J Clust Sci 24, 49–60 (2013). https://doi.org/10.1007/s10876-012-0513-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-012-0513-8