Abstract
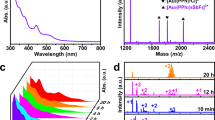
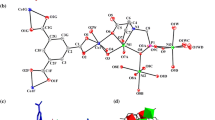
We report on the synthesis, stability, and photoluminescence (PL) properties of triphenylphosphine (PPh3)-stabilized PdAu10(PPh3)8Cl2 cluster, which is a mono-Pd-doped cluster of the well-studied Au11(PPh3)8Cl2 cluster. The PdAu10(PPh3)8Cl2 cluster was synthesized by simultaneously reducing two different metal complexes; AuCl(PPh3) and Pd(PPh3)4. Experimental evaluation of the stability showed that PdAu10(PPh3)8Cl2 is more stable against degradation in solution than the monometal Au11(PPh3)8Cl2 cluster. PL measurements revealed that PdAu10(PPh3)8Cl2 exhibits PL at 950 nm with a quantum yield of 1.5 × 10−3, which has not been observed for the monometal Au11(PPh3)8Cl2 cluster. The results indicate that Pd doping is a powerful method to produce clusters with higher stability and different physical properties than the monometal Au:PPh3 clusters.





Similar content being viewed by others
References
M. McPartlin, R. Mason, and L. Malatesta (1969). Chem. Commun. 334.
F. A. Vollenbroek, J. J. Bour, and J. W. A. van der Velden (1980). Rec. Trav. Chim. Pays-Bas 99, 137.
M. Schulz-Dobrick and M. Jansen (2007). Z. Anorg. Allg. Chem. 633, 2326.
G. H. Woehrle, M. G. Warner, and J. E. Hutchison (2002). J. Phys. Chem. B 106, 9979.
Y. Yang and S. Chen (2003). Nano Lett. 3, 75.
Y. Shichibu, Y. Negishi, T. Tsukuda, and T. Teranishi (2005). J. Am. Chem. Soc. 127, 13464.
Y. Liu, H. Tsunoyama, T. Akita, and T. Tsukuda (2009). J. Phys. Chem. C 113, 13457.
M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck, and H. Häkkinen (2008). Proc. Natl. Acad. Sci. USA 105, 9157.
C. E. Briant, B. R. C. Theobald, J. W. White, L. K. Bell, D. M. P. Mingos, and A. J. Welch (1981). J. Chem. Soc. Chem. Commun. 5, 201.
B. K. Teo, X. Shi, and H. Zhang (1992). J. Am. Chem. Soc. 114, 2743.
G. Schmid, R. Pfeil, R. Boese, F. Bandermann, S. Meyer, G. H. M. Calis, and J. W. A. van der Velden (1981). Chem. Ber. 114, 3634.
G. Schmid (1992). Chem. Rev. 92, 1709.
H.-G. Boyen, G. Kästle, F. Weigl, B. Koslowski, C. Dietrich, P. Ziemann, J. P. Spatz, S. Riethmüller, C. Hartmann, M. Möller, G. Schmid, M. G. Garnier, and P. Oelhafen (2002). Science 297, 1533.
T. Inomata and K. Konishi (2003). Chem. Commun. 1282.
R. Balasubramanian, R. Guo, A. J. Mills, and R. W. Murray (2005). J. Am. Chem. Soc. 127, 8126.
C. A. Fields-Zinna, M. C. Crowe, A. Dass, J. E. F. Weaver, and R. W. Murray (2009). Langmuir 25, 7704.
Y. Negishi, W. Kurashige, Y. Niihori, T. Iwasa, and K. Nobusada (2010). Phys. Chem. Chem. Phys. 12, 6219.
H. Qian, E. Barry, Y. Zhu, and R. Jin (2011). Acta Phys. Chim. Sin. 27, 513.
Y. Negishi, K. Igarashi, K. Munakata, W. Ohgake, and K. Nobusada (2012). Chem. Commun. 48, 660.
D.-e. Jiang and S. Dai (2009). Inorg. Chem. 48, 2720.
K. L. Craighead, A. M. P. Felicissimo, D. A. Krogstad, L. T. J. Nelson, and L. H. Pignolet (1993). Inorg. Chim. Acta. 212, 31.
M. Laupp and J. Strähle (1994). Angew. Chem. Int. Ed. Engl. 33, 207.
B. K. Teo and H. Zhang (1995). Coord. Chem. Rev. 143, 611.
M. Walter and M. Moseler (2009). J. Phys. Chem. C 113, 15834.
A. Seilmeier, B. Kopainsky, and W. Kaiser (1980). Appl. Phys. 22, 355.
Y. Yanagimoto, Y. Negishi, H. Fujihara, and T. Tsukuda (2006). J. Phys. Chem. B. 110, 11611.
B. K. Teo, H. Zhang, and X. Shi (1994). Inorg. Chem. 33, 4086.
B. K. Teo and H. Zhang (2000). J. Organomet. Chem. 614–615, 66.
B. K. Teo and H. Zhang (2001). J. Cluster Sci. 12, 349.
J. Zheng, J. T. Petty, and R. M. Dickson (2003). J. Am. Chem. Soc. 125, 7780.
J. Zheng, C. Zhang, and R. M. Dickson (2004). Phys. Rev. Lett. 93, 077402.
Y. Negishi, K. Nobusada, and T. Tsukuda (2005). J. Am. Chem. Soc. 127, 5261.
M.L. Tran, A.V. Zvyagin, and T. Plakhotnik (2006). Chem. Commun. 2400.
C.-C. Huang, Z. Yang, K.-H. Lee, and H.-T. Chang (2007). Angew. Chem. Int. Ed. Engl. 119, 6948.
C.-A. J. Lin, T.-Y. Yang, C.-H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J.-L. Shen, H.-H. Wang, H.-I. Yeh, W. J. Parak, and W. H. Chang (2009). ACS Nano 3, 395.
Y. Shichibu and K. Konishi (2010). Small 6, 1216.
Acknowledgments
The ESI–MS analysis was supported by the Collaborative Research Program of Institute for Chemical Research, Kyoto University. This study was financially supported by a Grant-in-Aid for Scientific Research (No. 21685003) and the “Nanotechnology Network” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurashige, W., Negishi, Y. Synthesis, Stability, and Photoluminescence Properties of PdAu10(PPh3)8Cl2 Clusters. J Clust Sci 23, 365–374 (2012). https://doi.org/10.1007/s10876-011-0437-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0437-8