Abstract
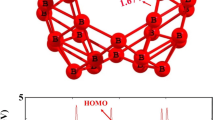
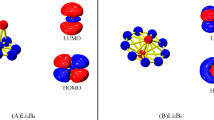
Endohedral derivatives of B16N16 nanocage (M@B16N16, M = Li+, Na+, K+, Mg2+, Ne, O2−, S2−, F−, Cl−) and its iso-electronic fullerne M@C32 have been employed to investigate the relation between the trapped atom/ion and electrophilicity of the B16N16 and C32 nanocages. The electrophilicity index, ω, of these endohedral nanocages has been evaluated from the ionization potential and the electron affinity computed by vertical ionization/affinity at the B3LYP/6-311++G(df,pd) level. Obtained results illustrate that the nature of trapped atom/ion affects HOMO-LUMO band gap, global electrophilicity indices and reactivity of B16N16 and C32 nanocages. Encapsulation B16N16 with different atom/ions may be a possible method for modifying HOMO-LUMO energy gap, electrophilicity and so chemical characteristics of and C32 nanocages.





Similar content being viewed by others
References
H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, and R. E. Smalley (1985). Nature 318, 162.
S. Iijima (1991). Nature 354, 56.
F. Jensen (1993). Chem. Phys. Lett. 209, 417.
I. Silaghi-Dumitrescu, F. Lara-Ochoa, P. Bishof, and I. Haiduc (1996). J. Mol. Struct. (Theochem) 367, 47.
N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie, and A. Zettl (1995). Science 269, 966.
M. Terrones, W. K. Hsu, H. Terrones, J. P. Zhang, S. Ramos, J. P. Hare, R. Castillo, K. Prassides, A. K. Cheetham, H. W. Kroto, and D. R. M. Walton (1996). Chem. Phys. Lett. 259, 568.
A. Loiseau, F. Williame, N. Demoncy, G. Hug, and H. Pascard (1996). Phys. Rev. Lett. 76, 4733.
Y. Saito and M. Maida (1999). J. Phys. Chem. A 103, 1291.
W. Koch and M. C. Holthausen A chemist’s guide to density functional theory (Wiley, Weinheim, 2000).
S. H. Xu, M. Y. Zhang, Y. Y. Zhao, B. G. Cheng, J. Zhang, and C. C. Sun (2006). Chem. Phys. Lett. 418, 297.
D. L. Strout (2000). J. Phys. Chem. A 104, 3364.
D. L. Strout (2004). Chem. Phys. Lett. 383, 95.
S. S. Alexandre, M. S. C. Mazzoni, and H. Chacham (1999). Appl. Phys. Lett. 75, 61.
S. S. Alexandre, R. W. Nunes, and H. Chacham (2002). Phys. Rev. B 66, 085.
H. S. Wu, X. H. Xu, F. Q. Zhang, and H. J. Jiao (2003). J. Phys. Chem. A 107, 6609.
H. S. Wu and H. J. Jiao (2004). Chem. Phys. Lett. 386, 369.
H. S. Wu, X. H. Xu, D. L. Strout, and H. J. Jiao (2005). J. Mol. Model. 12, 1.
A. E. Reed, L. A. Curtiss, and F. Weinhold (1988). Chem. Rev. 88, 899.
F. Weinhold and C. R. Landis (2001). Chem. Educ. Res. Pract. Eur. 2, 91.
F. Weinhold Natural bond orbital methods. in P. V. R. Schleyer, N. L. Allinger, T. Clark, J. Gasteiger, and P. A. Kollman (eds.), Encyclopedia of computational chemistry (Wiley, Chichester, UK, 1998).
F. Weinhold NBO 5.0 program manual. Theoretical chemistry institute and Department of chemistry (University of Wisconsin, Madison, WI, 2001), p. 53706.
A. R. Oliaey, A. Boshra, and M. Khavary (2010). Physica E 42, 2314.
V. Pophristic and L. Goodman (2001). Nature 411, 565.
L. Goodman, V. Pophristic, and F. Weinhold (1999). Acc. Chem. Res. 32, 983.
A. E. Reed and F. Weinhold (1985). J. Am. Chem. Soc. 107, 1919.
A. E. Reed and F. Weinhold (1986). J. Am. Chem. Soc. 108, 3586.
I. V. Alabugin, M. Manoharan, S. Peabody, and F. Weinhold (2003). J. Am. Chem. Soc. 125, 5973.
A. E. Reed, F. Weinhold, L. A. Curtiss, and D. J. Pochatko (1986). J. Chem. Phys. 86, 5687.
A. E. Reed and Pv. R. Schleyer (1987). J. Am. Chem. Soc. 109, 7362.
U. Salzner and P. R. Schleyer (1993). J. Am. Chem. Soc. 115, 10231.
D. Suárez, T. L. Sordo, and J. A. Sordo (1996). J. Am. Chem. Soc. 118, 9850.
N. K. Banavali and A. D. MacKerell Jr (2001). J. Am. Chem. Soc. 123, 6747.
E. D. Glendening, R. Faust, A. Streitwieser, K. P. C. Vollhardt, and F. Weinhold (1993). J. Am. Chem. Soc. 115, 10952.
M. Bruschi, M. G. Giuffreda, and H. P. Lüthi (2002). Chem. Eur. J. 8, 4216.
L. Fenga, Y. Lua, J. Kongb, and Zh. Suc (2011). Comput. Theor. Chem. 964, 56.
B. Napolion and Q. L. Williams (2010). Chem. Phys. Lett. 490, 210.
X. Cui, J.-F. Jia, B.-S. Yang, P. Yang, and H.-Sh. Wu (2010). J. Mol. Struct. (Theochem) 953, 1.
X. Cui, B.-Sh. Yang, and H.-S. Wu (2010). J. Mol. Struct. (Theochem) 941, 144.
B. Yin, G. Wang, N. Sa, and Y. Huang (2008). J. Mol. Model. 14, 789.
R. Li, L.-H. Gan, L. Lin, J.-Q. Zhao, and J. Liu (2009). J. Mol. Struct. (Theochem) 911, 75.
N. Karachi, A. Boshra, and S. Jadidi (2011). Struct. Chem. 22, 805.
V. Nirmala and P. Kolandaivel (2007). J. Mol. Struct. (Theochem) 817, 137.
X. Song, Sh. Liu, H. Yan, and Zh. Gan (2009). Physica E 41, 626.
P. A. Gauden and M. Wisniewski (2010). Catal. Today 150, 147.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, J. A. Pople, in: GAUSSIAN 98, Gaussian, Inc., Pittsburgh, PA (1998).
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
J. P. Perdew, K. Burke, and M. Ernzerhof (1996). Phys. Rev. Lett. 77, 3865.
J. P. Perdew, K. Burke, and M. Ernzerhof (1997). Phys. Rev. Lett. 78, 1396.
C. Adamo and V. Barone (1999). J. Chem. Phys. 110, 6158.
E. D. Glendening, A. E. Reed, J. E. Carpenter, and F. Weinhold NBO Version 3.1 (University of Wisconsin, Madison, TCI, 1998).
C.-G. Zhan, J. A. Nichols, and D. A. Dixon (2003). J. Phys. Chem. A 107, 4184.
R. G. Parr, L. von Szentpaly, and S. Liu (1999). J. Am. Chem. Soc. 121, 1922.
V. S. Gurin, Fuller. Nanotub. Car. N. 13, Suppl 1 (2005).
J. Almlof, T. Helgaker, and P. R. Taylor (1988). J. Phys. Chem. 92, 3029.
T. Clark, J. Chandrasekhar, G. W. Spitznagel, and P. R. Schleyer (1983). J. Comput. Chem. 4, 294.
T. John, S. Dennis, and H. Shinohara (1998). Appl. Phys. A 66, 243.
C.-Y. Zhang, H.-Sh. Wu, and H. Jiao (2007). J. Mol. Model. 13, 499.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boshra, A., Jadidi, S., Goudarzi, M. et al. DFT Study of Endohedral Atoms Effect on Electrophilicity of B16N16 Boron Nitride Nanocage: Comparative Analyses. J Clust Sci 23, 297–310 (2012). https://doi.org/10.1007/s10876-011-0430-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0430-2