Abstract
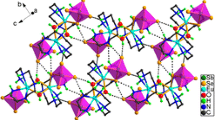
A series of 1-D lanthanide coordination polymers [Ln(μ3-OH)(pybz)(pa)] n (Ln = Er (1), Tb (2), Gd (3), Hpybz = 4-pyridin-4-yl-benzoic acid, Hpa = 2-picolinic acid) based on [Ln4(μ3-OH)4] cluster units have been hydrothermally synthesized and characterized by single crystal X-ray diffraction, IR, elemental analysis, and thermogravimetric analysis. X-ray crystal structure analyses reveal that 1–3 are isomorphous with tetragonal space group P \( \overline{4} \)21c and comprise tetranuclear Ln–O clusters, in which four Ln3+ centers are joined together by four μ3-bridging hydroxyl groups to form cubane-like [Ln4(μ3-OH)4]8+ cores that are further linked by four μ3-pa− ligands to produce 1-D chains along the c-axis.
Graphical Abstract
A series of 1-D lanthanide coordination polymers [Ln(μ3-OH)(pybz)(pa)] n (Ln = Er (1), Tb (2), Gd (3), Hpybz = 4-pyridin-4-yl-benzoic acid, Hpa = 2-picolinic acid) based on [Ln4(μ3-OH)4] cubanes have been hydrothermally synthesized. For this compound, tetranuclear cubane-like clusters [Ln4(μ3-OH)4]8+ composed of four Ln3+ centers as building blocks are further assembled into 1-D chains along the c-axis.







Similar content being viewed by others
References
R. Sessoli, D. Gatteschi, A. Caneschi, and M. A. Novak (1993). Nature 365, 141.
D. Gatteschi, A. Caneschi, R. Sessoli, and A. Cornia (1996). Chem. Soc. Rev. 25, 101.
A. P. Alivisatos (1996). Science 271, 933.
D. Gatteschi, L. Pardi, A. L. Barra, A. Müller, and J. Döring (1991). Nature 354, 463.
A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud, and G. Christou (2004). Angew. Chem. Int. Ed. 43, 2117.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, C. H. Yan, and G. X. Xu (2000). Angew. Chem. Int. Ed. 39, 2169.
R. Y. Wang, H. Liu, M. D. Carducci, T. Z. Jin, C. Zheng, and Z. P. Zheng (2001). Inorg. Chem. 40, 2743.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, and C. H. Yan (2000). New J. Chem. 24, 251.
V. Baskar and P. W. Roesky (2006). Dalton Trans. 676.
F. N. Shi, L. Cunha-Silva, T. Trindade, F. A. Paz, and J. Rocha (2009). Cryst. Growth Des. 9, 2098.
R. Y. Wang, H. D. Selby, H. Liu, M. D. Carducci, T. Z. Jin, Z. P. Zheng, J. W. Anthis, and R. J. Staples (2002). Inorg. Chem. 41, 278.
J. C. Plakatouras, L. Baxter, M. B. Hursthouse, K. M. Abdul Malik, J. McAleese, and S. R. Drake (1994). Chem. Soc. Chem. Commun. 2455.
X. M. Chen, Y. L. Wu, Y. X. Tong, Z. D. Sun, and N. Hendrickson (1997). Polyhedron 16, 4265.
T. Dube, S. Gambarotta, and G. Yap (1998). Organometallics 17, 3967.
J. W. Cheng, J. Zhang, and G. Y. Yang (2006). Angew. Chem. Int. Ed. 45, 73.
J. W. Cheng, S. T. Zheng, and G. Y. Yang (2008). Chem. Eur. J. 14, 88.
M. B. Zhang, J. Zhang, and G. Y. Yang (2005). Angew. Chem. Int. Ed. 44, 1385.
J. W. Cheng, J. Zhang, and G. Y. Yang (2007). Inorg. Chem. 46, 10261.
G. M. Sheldrick SHELXS97, Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXL97, Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
Acknowledgments
The authors are thankful for the financial supports from the National Natural Science Fund for Distinguished Young Scholars of China (no. 20725101), the NNSF of China (no. 50872133), the 973 Program (no. 2006CB932904), the NSF of Fujian Province (nos. E0510030 and 2008F3120), and the Knowledge Innovation Program from CAS (no. KJCX2.YW.H01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, ZL., Fang, WH. & Yang, GY. A Series of 1-D Lanthanide Coordination Polymers Based on [Ln4(μ3-OH)4] (Ln = Er, Tb, Gd) Cluster Units. J Clust Sci 20, 725–733 (2009). https://doi.org/10.1007/s10876-009-0276-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-009-0276-z