Abstract
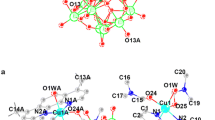
Two new phosphatotungstates containing Keggin clusters, [Cu(2,2′-bipy)2]5[PW12O40] · 2H2O (1) (2,2′-bipy = 2,2′-bipyridine) and (Hpip)3[PW12O40] (2) (pip = piperazine) have been hydrothermally synthesized and characterized by IR, element analysis and cyclic voltammogram. Compound 1 consists of one discrete Keggin polyanion [PW12O40]5−, five isolated complex cations [Cu(2,2′-bipy)2]+ and two water molecules. The organic moieties exhibit regular packing with offset aromatic–aromatic interactions between the bipys, leading to a compact supramolecular framework structure. Compound 2 is made up of one discrete Keggin polyanion [PW12O40]3− and three pip cations. Compounds 1 and 2 were employed to fabricate bulk-modified carbon paste electrode to research on their electrochemistry properties. Their electrochemical behaviors and electrocatalysis that 1- and 2-CPEs have electrocatalytic activities toward the oxidation of nitrite. Compound 1 is in the orthorhombic system, space group Pna21, with a = 28.1928(9), b = 21.5479(6), c = 19.9088(6) Å, V = 12,094.5(6) Å3 and Z = 4. Compound 2 is in the rhombohedral system, space group R\( \overline{3} \)c, with a = 17.9191(5), c = 23.7439(9) Å, V = 6,602.6(4) Å3 and Z = 6.





Similar content being viewed by others
References
C. L. Hill (1998). Chem. Rev. 98, 1.
L. Xu, M. Lu, B. B. Xu, Y. G. Wei, Z. H. Peng, and D. R. Powell (2002). Angew. Chem. Int. Ed. Engl. 114, 4303.
C. L. Hill (2004). Angew. Chem. Int. Ed. Engl. 43, 402.
T. Yamase (1998). Chem. Rev. 98, 307.
K. F. Aguey-Zinsou, P. V. Bernhardet, U. Kappler, and A. G. McEwan (2003). J. Am. Chem. Soc. 125, 530.
M. T. Pope and A. Müller (1991). Angew. Chem. Int. Ed. Engl. 30, 34.
D. L. Long, E. Burkholder, and L. Cronin (2007). Chem. Soc. Rev. 36, 105.
P. J. Hagrman, D. Hagrman, and J. Zubieta (1999). Angew. Chem. Int. Ed. Engl. 38, 2638.
P. Q. Zheng, Y. P. Ren, L. S. Long, R. B. Huang, and L. S. Zheng (2005). Inorg. Chem. 44, 1190.
H. Y. An, E. B. Wang, D. R. Xiao, Y. G. Li, Z. M. Su, and L. Xu (2006). Angew. Chem. Int. Ed. Engl. 45, 904.
G. Y. Luan, Y.G. Li, S. T. Wang, E. B. Wang, Z. B. Han, C. W. Hu, N. H. Hu, and H. Q. Jia (2003). Dalton Trans. 233.
W. B. Yang, C. Z. Lu, and H. H. Zhuang (2002). J. Chem. Soc., Dalton Trans. 2879.
B. Z. Lin, Z. Li, L. W. He, L. Bai, X. F. Huang, and Y. L. Chen (2007). Inorg. Chem. Commun. 10, 600.
R. N. Devi, E. Burkholder, and J. Zubieta (2003). Inorg. Chim. Acta. 348, 150.
L. Lisnard, A. Dolbecq, P. Mialane, J. Marrot, and F. Sécheresse (2004). Inorg. Chim. Acta. 357, 845.
T. H. Li, J. Lu, Sh. Y. Gao, and R. Cao (2007). Inorg. Chem. Commun. 10, 551.
C. L. Pan, J. Q. Xu, G. H. Li, D. Q. Chu, and T. G. Wang (2003). Eur. J. Inorg. Chem. 1514.
M. T. Pope, Heteropoly and Isopoly Oxometalates (Springer-Verlag, New York, 1983).
M. Sadakane and E. Steckhan (1998). Chem. Rev. 98, 219.
X. L. Wang, Z. H. Kang, E. B. Wang, and C. W. Hu (2002). Mater. Lett. 56, 393.
B. X. Dong, J. Peng, A. X. Tian, J. Q. Sha, L. Li, and H. S. Liu (2007). Electrochim. Acta. 52, 3804.
G. M. Sheldrick, SADABS (University of Göttingen, Germany, 1996).
G. M. Sheldrick, SHELX97 (University of Göttingen, Germany, 1997).
L. W. He, B. Z. Lin, X. Z. Liu, X. F. Huang, and Y. L. Feng (2008). Solid State Sci. 10, 237.
J. X. Chen, T. Y. Lan, Y. B. Huang, C. X. Wei, Z. S. Li, and Z. C. Zhang (2006). J. Solid State Chem. 179, 1904.
S. Chang, C. Qin, E. B. Wang, Y. G. Li, and X. L. Wang (2006). Inorg. Chem. Commun. 9, 727.
Z. Li, B. Z. Lin, J. F. Zhang, F. Geng, G. H. Han, and Pei-De Liu (2006). J. Mol. Struct. 783, 176.
J. P. Wang, X. Y. Duan, and J. Y. Niu (2004). J. Mol. Struct. 692, 17.
I. D. Brown and D. Altermatt (1985). Acta Crystallogr. Set. B. 41, 244.
B. Z. Lin, and S. X. Liu (2002) Chem. Commun. 2126.
J. Y. Niu, Y. Shen, and J. P. Wang (2005). J. Mol. Struct. 733, 19.
C. M. Liu, D. Q. Zhang, and D. B. Zhu (2003). Cryst. Growth Des. 3, 363.
J. Y. Niu, D. J. Guo, J. W. Zhao, and J. P. Wang (2004). New J. Chem. 28, 980.
B. Z Lin, Y. M. Chen, and P. D. Liu (2003) Dalton Trans. 2474.
T. Y. Lan, J. X. Chen, C. X. Wei, Z. S. Li, Y. B. Huang, W. J. Zhang, and Z. C. Zhang (2006). Chin. J. Struct. Chem. 25, 368.
Z. Li, B. Z. Lin, G. H. Han, F. Geng, and P. D. Liu (2005). Chin. J. Struct. Chem. 24, 608.
Z. G. Han, Y. L. Zhao, J. Peng, H. Y. Ma, Q. Liu, E. B. Wang, and N. H. Hu (2004). J. Solid State Chem. 177, 4325.
J. E. Toth and F. C. Anson (1989). J. Am. Chem. Soc. 111, 219.
Acknowledgments
The authors thank the NSF of Fujian Province (nos. E0420001, 2005HZ01-4) and NCETFJ for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bai, L., Lin, BZ., Huang, XF. et al. Hydrothermal Synthesis, Crystal Structures and Electrochemical Properties of Two Phosphatotungstates Containing Keggin Clusters, [Cu(2,2′-bipy)2]5[PW12O40] · 2H2O and (Hpip)3[PW12O40]. J Clust Sci 19, 561–572 (2008). https://doi.org/10.1007/s10876-008-0202-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-008-0202-9