Abstract
The mixed-valence 14-vanadogermanate [GeVV 12VIV 2O40]8− (1) has been synthesized and characterized in solution by 51V-NMR, UV–vis and electrochemistry and in the solid state by IR, magnetism, EPR, XPS and elemental analysis. Single-crystal X-ray analysis was carried out on K2Na6[GeVV 12VIV 2O40]·10H2O (KNa-1), which crystallizes in the orthorhombic system, space group Immm, with a=10.9623(3) Å, b=11.6205(3) Å, c=20.2658(5) Å, and Z=2. Polyanion 1 is composed of a central GeIVO6 octahedron which is surrounded by a total of 14 VO6 octahedra. Vanadium-51 NMR in solution results in three peaks with intensity ratio of 8:4:2 which is in complete agreement with the solid state structure. The presence of two VIV centers was established by UV–vis, electrochemistry, magnetism, EPR, XPS and elemental analysis. Electrochemistry revealed that the two VIV-centers in 1 are oxidized through a single well-defined step, which does not split with changes in scan rate or pH. Polyanion 1 is also an active two-electron oxidation catalyst for the coenzyme NADH at pH 8, unprecedented in polyoxometalate chemistry. Magnetic susceptibility, magnetization and EPR data on KNa-1 complement the X-ray and electrochemistry results by confirming the presence of two unpaired electrons per molecule of 1. The two VIV ions possessing the spin are very weakly coupled, essentially acting as two well-isolated S=1/2 ions. The observed g-value of 1.977 from EPR and magnetic susceptibility measurements is consistent with literature reported value for a VIV ion, suggesting a possible ground state of \(3d_{x^{2}-y^{2}}.\) XPS measurements on KNa-1 also confirmed the coexistence of VV and VIV in 1.










Similar content being viewed by others
References
(a) J. J. Berzelius (1826). Pogg. Ann. 6, 369. (b) J. F. Keggin (1933). Nature 131, 908. (c) J. F. Keggin (1934). Proc. Roy. Soc. A 144, 75
(a) M. T. Pope, Heteropoly and Isopoly Oxometalates (Springer, Berlin, 1983). (b) M. T. Pope and A. Müller (1991). Angew. Chem. Int. Ed. 30, 34.
(a) M. T. Pope and A. Müller (eds.), Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity (Kluwer, Dordrecht, 1994). (b) C. L. Hill (ed.), Chemical Reviews, Polyoxometalates (1998). (c) M. T. Pope and A. Müller (eds.) Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications (Kluwer, Dordrecht, 2001). (d) T. Yamase and M. T. Pope (eds.) Polyoxometalate Chemistry for Nano-Composite Design (Kluwer, Dordrecht, 2002). (e) M. T. Pope (2003). Comp. Coord. Chem. II 4, 635. (f) C. L. Hill (2003). Comp. Coord. Chem. II 4, 679. (g) J. J. Borrás-Almenar, E. Coronado, A. Müller, and M. T. Pope (eds.), Polyoxometalate Molecular Science (Kluwer, Dordrecht, 2004). (h) N. Casan-Pastor and P. Gomez-Romero (2004). Fron. Biosci. 9, 1759
(a) L. Chen, F. Jiang, Z. Lin, Y. Zhou, C. Yue, and M. Hong (2005). J. Am. Chem. Soc. 127, 8588. (b) M. I. Khan, S. Ayesh, R. J. Doedens, M. H. Yu, and C. J. O’Connor (2005). Chem. Commun. 4658 and references therein
(a) A. R. Gaspar, D. V. Evtuguin, and C. P. Neto (2004). Ind. Eng. Chem. Res. 43, 7754. (b) A. Bose, P. He, C. Liu, B. D. Ellman, R. J. Twieg, and S. D. Huang (2002). J. Am. Chem. Soc. 124, 4. (c) A. M. Khenkin, L. Weiner, and R. Neumann (2005). J. Am. Chem. Soc. 127, 9988
(a) A. Müller, H. Reuter, and S. Dillinger (1995). Angew. Chem. Int. Ed. 34, 2328. (b) A. Müller, A. M. Todea, J. van Slageren, M. Dressel, H. Bögge, M. Schmidtmann, M. Luban, L. Engelhardt, and M. Rusu (2005). Angew. Chem. Int. Ed. 44, 3857. (c) A. Müller, and P. Kögerler (1999). Coord. Chem. Rev. 182, 3. (d) A. Müller, J. Meyer, H. Bögge, A. Stammler, and A. Botar (1998). Chem. Eur. J. 4, 1388. (e) A. Müller, R. Sessoli, E. Kickemeyer, H. Bögge, J. Meyer, D. Gatteschi, L. Pardi, J. Westphahl, K. Hovemaier, R. Rohlfing, J. Döring, F. Hellweg, C. Beugholt, and M. Schmidtmann (1997). Inorg. Chem. 36, 5239
A. Müller, J. Döring, M. I. Khan, and V. Wittneben (1991). Angew. Chem. Int. Ed. 30, 210
(a) C. M. Flynn Jr. and M. T. Pope (1970). J. Am. Chem. Soc. 92, 85. (b) A. Kobayashi and Y. Sasaki (1975). Chem. Lett. 1123. (c) K. Nagai, H. Ichida, and Y. Sasaki (1986). Chem. Lett. 1267
H. T. Evans Jr. and J. A. Konnert (1978). Am. Mineral. 63, 863
G. M. Sheldrick (1996). SADABS (University of Göttingen, Germany)
B. Keita, F. Girard, L. Nadjo, R. Contant, J. Canny and M. Richet (1999). J. Electroanal. Chem. 478, 76
(a) S. G. Vulfson (ed.), Molecular Magnetochemistry (Gordon and Breach Science, Newark, 1998). (b) U. Kortz, S. Nellutla, A. C. Stowe, N. S. Dalal, J. van Tol, and B. S. Bassil (2004). Inorg. Chem. 43, 144. (c) U. Kortz, S. Nellutla, A. C. Stowe, N. S. Dalal, U. Rauwald, W. Danquah, and D. Ravot (2004). Inorg. Chem. 43, 2308
I. D. Brown and D. Altermatt (1985). Acta Cryst. B41, 244
B. Keita, I.-M. Mbomekalle, L. Nadjo, and C. Haut (2004). Electrochem. Commun. 6, 978
B. Keita, I.-M. Mbomekalle, L. Nadjo, P. de Oliveira, A. Ranjbari, and R. Contant (2005). C. R. Chimie 8, 1057
B. Keita, K. Essaadi, L. Nadjo, R. Contant and Y. Justum (1996). J. Electroanal. Chem. 404, 271
(a) O. Kahn (ed.), Molecular Magnetism (VCH, New York, 1993). (b) R. L. Carlin (ed.), Magnetochemistry (Springer, Berlin, 1986)
(a) S.-T. Zheng, J. Zhang, and G.-Y. Yang (2005). Inorg. Chem. 44, 2426. (b) S.-T. Zheng, J. Zhang, and G.-Y. Yang (2004). Eur. J. Inorg. Chem. 2004. (c) A.-L. Barra, D. Gatteschi, L. Pardi, A. Müller, and J. Döring (1992). J. Am. Chem. Soc. 114, 8509. (d) A. Müller, J. Döring, and H. Bögge (1991). J. Chem. Soc., Chem. Comm. 273
(a) Y.-G. Li, Y. Lu, G.-Y. Luan, E.-B. Wang, Y.-B. Duan, C.-W. Hu, N.-H. Hu, and H.-Q. Jia (2002). Polyhedron 21, 2601. (b) I. Sougandi, R. Venkatesan, and P. S. Rao (2003). J. Phys. Chem. Solids, 64, 1231. (c) V. K. Jain, and V. Putcha (1980). J. Chem. Phys. 73, 30. (d) A. Abragam, and B. Bleaney (eds.), Electron Paramagnetic Resonance of Transition Ions (Clarendon Press, Oxford, 1970). (e) I. Siegel (1964). Phys. Rev. 134, A193
M. Demeter, M. Neumann, and W. Reichelt (2000). Surf. Sci. 41, 454
M. Demeter, Spectroscopic Study of Transition Metal Compounds (Ph.D. thesis, University of Osnabrück, 2001)
Acknowledgments
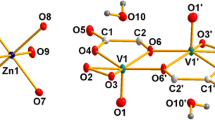
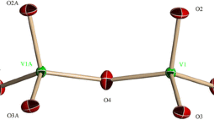
U. K. thanks the International University Bremen for research support. We would like to thank Mr. Donny Magana of Florida State University for helpful discussions, and the National Science Foundation grant NIRT-DMR 1103290 for partial financial support. This work was also supported by the CNRS (UMR 8000) and the University Paris-Sud XI. Figures 1, 2 were generated by Diamond Version 3.1 (copyright Crystal Impact GbR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Michael T. Pope on the occasion of his retirement.
Rights and permissions
About this article
Cite this article
Bi, LH., Kortz, U., Dickman, M. et al. Polyoxoanion with Octahedral Germanium(IV) Hetero Atom: Synthesis, Structure, Magnetism, EPR, Electrochemistry and XPS Studies on the Mixed-Valence 14-Vanadogermanate [GeVV 12VIV 2O40]8− . J Clust Sci 17, 143–165 (2006). https://doi.org/10.1007/s10876-006-0048-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-006-0048-y