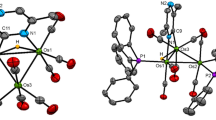
In toluene at reflux temperatures [Ru3(CO)12] and 7-SMe2-nido-7-CB10H12 give the charge-compensated cluster complex [1-SMe2-2,2-(CO)2-7,11-(μ-H)2-2,7,11-Ru2(CO)6-closo-2,1-RuCB10H8] (1) . Treatment of 1 with dppm in THF affords [1-SMe2-2,2-(CO)2-7,11-(μ-H)2-2,7,11-Ru2(μ -dppm)(CO)4-closo-2,1-RuCB10H8] (2) [dppm = bis(diphenylphosphino)methane; THF = tetrahydrofuran]. The latter complex on heating in THF with [\({\hbox{NBu}^{n}}_{4}\)]F yields the salt [\({\hbox{NBu}^{n}}_{4}\)][1-SMe-2,2-(CO)2-7,11-(μ-H)2-2,7,11-Ru2(μ -dppm)(CO)4-closo-2,1-RuCB10H8] (3). Reaction of 3 with [AuCl(PPh3)] and Tl[PF6] gives the neutral zwitterionic complex [1-S(Me)Au (PPh3)-2,2-(CO)2-7,11-(μ-H)2-2,7,11-Ru2(μ-dppm)(CO)4-closo-2,1-RuCB10H8] (4). The structures of 1, 3 and 4 were determined by single-crystal X-ray diffraction studies.
Similar content being viewed by others
References
(a) R. N. Grimes, in G. Wilkinson, E. W. Abel, and F. G. A. Stone (eds.), Comprehensive Organometallic Chemistry (Pergamon Press, Oxford, United Kingdom, 1982), Vol. 1, Section 5.5. (b) R. N. Grimes, in E. W. Abel, F. G. A. Stone, and G. Wilkinson (eds.), Comprehensive Organometallic Chemistry II (Pergamon Press, Oxford, United Kingdom, 1995), Vol. 1 (Ed. C. E. Housecroft), Chapter 9. (c) R. N. Grimes (2000). Coord. Chem. Rev. 200–202, 773
T. D. McGrath and F. G. A. Stone (2005). Adv. Organomet. Chem (in press)
T. D. McGrath F. G. A. Stone (2004) J. Organomet. Chem 689 3891 Occurrence Handle10.1016/j.jorganchem.2004.05.034 Occurrence Handle1:CAS:528:DC%2BD2cXhtVWgt7vJ
T. Jelínek J. Plesek S. Hermánek B. Stíbr (1985) Collect. Czech. Chem. Comm 50 1376
F. Piacenti M. Bianchi P. Frediani E. Benedetti (1971) Inorg. Chem 10 2759 Occurrence Handle10.1021/ic50106a027 Occurrence Handle1:CAS:528:DyaE38XitVOqtQ%3D%3D
S. R. Drake R. Khattar (1988) Organomet. Synth 4 234
W. Quintana L. G. Sneddon (1990) Inorg. Chem 29 3242 Occurrence Handle10.1021/ic00342a039 Occurrence Handle1:CAS:528:DyaK3cXkvFOlt7Y%3D
V. N. Lebedev D. F. Mullica E. L. Sappenfield F. G. A. Stone (1996) Organometallics 15 1669 Occurrence Handle10.1021/om950725p Occurrence Handle1:CAS:528:DyaK28Xht1ylurs%3D
S. A. Brew F. G. A. Stone (1993) Adv. Organomet. Chem 35 135 Occurrence Handle1:CAS:528:DyaK2cXlsVGrsQ%3D%3D
D. F. Mullica E. L. Sappenfield F. G. A. Stone S. F. Woollam (1994) Organometallics 13 157 Occurrence Handle10.1021/om00013a026 Occurrence Handle1:CAS:528:DyaK2cXitFyjs78%3D
S. Du, A. Franken, P. A. Jelliss, J. A. Kautz, F. G. A. Stone, and P.-Y. Yu (2001). J. Chem. Soc., Dalton Trans. 1846
D. D. Ellis A. Franken F. G. A. Stone (1999) Organometallics 18 2362 Occurrence Handle10.1021/om990133x Occurrence Handle1:CAS:528:DyaK1MXjsVKhs7o%3D
R. Usón R. Laguna (1986) Organomet. Synth 3 322
APEX 2, version 1.0-5, Bruker AXS, Madison, WI, 2003
SHELXTL version 6.12, Bruker AXS, Madison, WI, 2001
Acknowledgements
We thank the Robert A. Welch Foundation for support (Grant AA-1201). The Bruker-Nonius X8 APEX diffractometer was purchased with funds received from the National Science Foundation Major Research Instrumentation Program (Grant CHE-0321214).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Professor F. Albert Cotton on the occasion of his 75th birthday, in appreciation of our long friendship and in recognition of his outstanding contributions to the study of complexes with metal–metal bonds.
Rights and permissions
About this article
Cite this article
McGrath, T.D., Stone, F.G.A. & Sukcharoenphon, K. Synthesis, Molecular Structure and Derivatization of the Triruthenacarborane Cluster Compound [1-SMe2-2,2-(CO)2-7,11-(Μ-H)2-2,7,11-{Ru2(CO)6}-closo-2,1-RuCB10H8]*. J Clust Sci 16, 201–215 (2005). https://doi.org/10.1007/s10876-005-4544-2
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10876-005-4544-2