Abstract
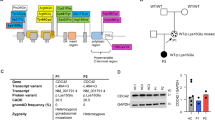
AIOLOS, a vital member of the IKAROS protein family, plays a significant role in lymphocyte development and function through DNA binding and protein–protein interactions. Mutations in the IKZF3 gene, which encodes AIOLOS, lead to a rare combined immunodeficiency often linked with infections and malignancy. In this study, we evaluated a 1-year-4-month-old female patient presenting with recurrent infections, diarrhea, and failure to thrive. Laboratory investigations revealed decreased T lymphocyte and immunoglobulin levels. Through whole-exome and Sanger sequencing, we discovered a de novo mutation in IKZF3 (NM_012481; exon 5 c.571G > C, p.Gly191Arg), corresponding to the third DNA-binding zinc finger region of the encoded protein AIOLOS. Notably, the patient with the AIOLOS G191R mutation showed reduced recent thymic emigrants in naïve CD4+T cells compared to healthy counterparts of the same age, while maintaining normal levels of Th1, Th2, Th17, Treg, and Tfh cells. This mutation also resulted in decreased switched memory B cells and lower CD23 and IgM expression. In vitro studies revealed that AIOLOS G191R does not impact the expression of AIOLOS but compromises its stability, DNA binding and pericentromeric targeting. Furthermore, AIOLOS G191R demonstrated a dominant-negative effect over the wild-type protein. This case represents the first reported instance of a mutation in the third DNA-binding zinc finger region of AIOLOS highlighting its pivotal role in immune cell functionality.




Similar content being viewed by others
Data Availability
The data analyzed in the current study is available from the corresponding author on reasonable request.
References
Staels F, Collignon T, Betrains A, Gerbaux M, Willemsen M, Humblet-Baron S, et al. Monogenic adult-onset inborn errors of immunity. Front Immunol. 2021;12: 753978.
Fischer A. Gene therapy for inborn errors of immunity: past, present and future. Nat Rev Immunol. 2023;23(6):397–408.
Elsink K, Huibers MM, Hollink IH, Simons A, Zonneveld-Huijssoon E, van der Veken LT, et al. Implementation of early next-generation sequencing for inborn errors of immunity: a prospective observational cohort study of diagnostic yield and clinical implications in Dutch genome diagnostic centers. Front Immunol. 2021;12: 780134.
Suspitsin EN, Guseva MN, Kostik MM, Sokolenko AP, Skripchenko NV, Levina AS, et al. Next generation sequencing analysis of consecutive Russian patients with clinical suspicion of inborn errors of immunity. Clin Genet. 2020;98(3):231–9.
John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–8.
Heizmann B, Kastner P, Chan S. The Ikaros family in lymphocyte development. Curr Opin Immunol. 2018;51:14–23.
Yamashita M, Morio T. Inborn errors of IKAROS and AIOLOS. Curr Opin Immunol. 2021;72:239–48.
Marke R, van Leeuwen FN, Scheijen B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2018;103(4):565.
Zhang X, Zhang X, Li X, Lv Y, Zhu Y, Wang J, et al. The specific distribution pattern of IKZF1 mutation in acute myeloid leukemia. J Hematol Oncol. 2020;13:1–5.
Hetemäki I, Kaustio M, Kinnunen M, Heikkilä N, Keskitalo S, Nowlan K, et al. Loss-of-function mutation in IKZF2 leads to immunodeficiency with dysregulated germinal center reactions and reduction of MAIT cells. Science Immunol. 2021;6(65):eabe3454.
Shahin T, Mayr D, Shoeb MR, Kuehn HS, Hoeger B, Giuliani S, et al. Identification of germline monoallelic mutations in IKZF2 in patients with immune dysregulation. Blood Adv. 2022;6(7):2444–51.
Gokhale AS, Gangaplara A, Lopez-Occasio M, Thornton AM, Shevach EM. Selective deletion of Eos (Ikzf4) in T-regulatory cells leads to loss of suppressive function and development of systemic autoimmunity. J Autoimmun. 2019;105: 102300.
Lentaigne C, Greene D, Sivapalaratnam S, Favier R, Seyres D, Thys C, et al. Germline mutations in the transcription factor IKZF5 cause thrombocytopenia. Blood. 2019;134(23):2070–81.
Lazarian G, Yin S, Ten Hacken E, Sewastianik T, Uduman M, Font-Tello A, et al. A hotspot mutation in transcription factor IKZF3 drives B cell neoplasia via transcriptional dysregulation. Cancer Cell. 2021;39(3):380-93.e8.
Ma S, Pathak S, Mandal M, Trinh L, Clark MR, Lu R. Ikaros and Aiolos inhibit pre-B-cell proliferation by directly suppressing c-Myc expression. Mol Cell Biol. 2010;30(17):4149–58.
Yamashita M, Kuehn HS, Okuyama K, Okada S, Inoue Y, Mitsuiki N, et al. A variant in human AIOLOS impairs adaptive immunity by interfering with IKAROS. Nat Immunol. 2021;22(7):893–903.
Kuehn HS, Chang J, Yamashita M, Niemela JE, Zou C, Okuyama K, et al. T and B cell abnormalities, pneumocystis pneumonia, and chronic lymphocytic leukemia associated with an AIOLOS defect in patients. J Exp Med. 2021;218(12): e20211118.
Kuehn HS, Sakovich IS, Niemela JE, Silva AAG, Stoddard JL, Polyakova EA, et al. Disease-associated AIOLOS variants lead to immune deficiency/dysregulation by haploinsufficiency and redefine AIOLOS functional domains. J Clin Investig. 2024;134(3): e172573.
Ding Y, Zhou L, Xia Y, Wang W, Wang Y, Li L, et al. Reference values for peripheral blood lymphocyte subsets of healthy children in China. J Allergy Clin Immunol. 2018;142(3):970-3.e8.
Zhao Q, Dai R, Li Y, Wang Y, Chen X, Shu Z, et al. Trends in TREC values according to age and gender in Chinese children and their clinical applications. Eur J Pediatr. 2022;181(2):529–38.
Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14(17):2146–60.
Caballero R, Setien F, Lopez-Serra L, Boix-Chornet M, Fraga MF, Ropero S, et al. Combinatorial effects of splice variants modulate function of Aiolos. J Cell Sci. 2007;120(Pt 15):2619–30.
Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16(8):2004–13.
Kohler S, Keil T, Alexander T, Thiel A, Swierzy M, Ismail M, et al. Altered naive CD4(+) T cell homeostasis in myasthenia gravis and thymoma patients. J Neuroimmunol. 2019;327:10–4.
Yamashita M, Morio T. AIOLOS variants causing immunodeficiency in human and mice. Front Immunol. 2022;13: 866582.
Apostolov A. Studying the posttranslational modifications of tran- scription factor Ikaros and their role in its function: Strasbourg. 2012
Kuehn HS, Chang J, Yamashita M, Niemela JE, Zou C, Okuy- ama K, et al. T and B cell abnormalities, pneumocystis pneumo- nia, and chronic lymphocytic leukemia associated with an AIO- LOS defect in patients. J Exp Med. 2021;218(12):e20211118. 27.
Funding
This study was supported by the National Natural Science Foundation of China (32360184), the Guizhou Provincial Science and Technology Projects (QKHPTRC-CXTD[2021]010, QKHZK-2022-YB641), the Collaborative Innovation Center of Chinese Ministry of Education (2020‑39) and the Guangdong Medical Research Foundation (B2022077).
Author information
Authors and Affiliations
Contributions
XQ.S was responsible for drafting the initial manuscript, analyzing the data, and creating the figures. ZC.D and P.H conceived the study and were in charge of reviewing and revising the manuscript. XL.C, AY.R, and XQ.S carried out flow cytometry, Western blotting (WB), and co-immunoprecipitation (CO-IP). R.C completed the TREC and KREC assays. X.D handled the Electrophoretic Mobility Shift Assay (EMSA). GL.Y and PP.Z managed the confocal microscopy. ZG.Y, CZ.Y, Y.C, and XD.Z reviewed and revised the manuscript. MY.H collected and evaluated key clinical information. Each author approved the final version of the manuscript and is accountable for all aspects of the research.
Corresponding authors
Ethics declarations
Ethics Approval
The legal guardian of the patient provided written informed consent and was briefed about the involvement in this research. The Affiliated Hospital of Zunyi Medical University's Ethics Committee granted approval for this study (Approval Number: KLL-2023–535). All procedures adhered to the pertinent guidelines and standards.
Consent to Participate
The patient's parents provided written informed consent.
Consent for Publication
Every author gave their approval for the final manuscript version.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, X., Cao, X., Huang, M. et al. Identification and Functional Analysis of a de novo IKZF3 Mutation in a Pediatric Patient with Combined Immunodeficiency. J Clin Immunol 44, 117 (2024). https://doi.org/10.1007/s10875-024-01706-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10875-024-01706-9