Abstract
Background
Deoxyribonuclease 1 like 3 (DNASE1L3) is a secreted enzyme that has been shown to digest the extracellular chromatin derived from apoptotic bodies, and DNASE1L3 pathogenic variants have been associated with a lupus phenotype. It is unclear whether interferon signaling is sustained in DNASE1L3 deficiency in humans.
Objectives
To explore interferon signaling in DNASE1L3 deficient patients. To depict the characteristic features of DNASE1L3 deficiencies in human.
Methods
We identified, characterized, and analyzed five new patients carrying biallelic DNASE1L3 variations. Whole or targeted exome and/or Sanger sequencing was performed to detect pathogenic variations in five juvenile systemic erythematosus lupus (jSLE) patients. We measured interferon-stimulated gene (ISG) expression in all patients. We performed a systematic review of all published cases available from its first description in 2011 to March 24th 2022.
Results
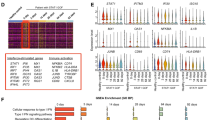
We identified five new patients carrying biallelic DNASE1L3 pathogenic variations, including three previously unreported mutations. Contrary to canonical type I interferonopathies, we noticed a transient increase of ISGs in blood, which returned to normal with disease remission. Disease in one patient was characterized by lupus nephritis and skin lesions, while four others exhibited hypocomplementemic urticarial vasculitis syndrome. The fourth patient presented also with early-onset inflammatory bowel disease. Reviewing previous reports, we identified 35 additional patients with DNASE1L3 deficiency which was associated with a significant risk of lupus nephritis and a poor outcome together with the presence of anti-neutrophil cytoplasmic antibodies (ANCA). Lung lesions were reported in 6/35 patients.
Conclusions
DNASE1L3 deficiencies are associated with a broad phenotype including frequently lupus nephritis and hypocomplementemic urticarial vasculitis with positive ANCA and rarely, alveolar hemorrhages and inflammatory bowel disease. This report shows that interferon production is transient contrary to anomalies of intracellular DNA sensing and signaling observed in Aicardi-Goutières syndrome or STING-associated vasculitis in infancy (SAVI).


Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
References
Belot A, Rice GI, Omarjee SO, Rouchon Q, Smith EMD, Moreews M, et al. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts. Lancet Rheumatol [Internet]. 2020 [cited 2020 Jan 16];0. Available from: https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(19)30142-0/abstract.
Belot A, Cimaz R. Monogenic forms of systemic lupus erythematosus: new insights into SLE pathogenesis. Pediatr Rheumatol Online J. 2012;10:21.
Omarjee O, Picard C, Frachette C, Moreews M, Rieux-Laucat F, Soulas-Sprauel P, et al. Monogenic lupus: dissecting heterogeneity. Autoimmun Rev. 2019;18:102361.
Briggs TA, Rice GI, Daly S, Urquhart J, Gornall H, Bader-Meunier B, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–31.
An J, Briggs TA, Dumax-Vorzet A, Alarcón-Riquelme ME, Belot A, Beresford M, et al. Tartrate-resistant acid phosphatase deficiency in the predisposition to systemic lupus erythematosus. Arthritis Rheumatol Hoboken NJ. 2017;69:131–42.
Crow YJ. Lupus: how much “complexity” is really (just) genetic heterogeneity? Arthritis Rheum. 2011;63:3661–4.
Belot A, Kasher PR, Trotter EW, Foray A-P, Debaud A-L, Rice GI, et al. Protein kinase cδ deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 2013;65:2161–71.
Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet. 2011;43:1186–8.
Özçakar ZB, Foster J, Diaz-Horta O, Kasapcopur O, Fan Y-S, Yalçınkaya F, et al. DNASE1L3 mutations in hypocomplementemic urticarial vasculitis syndrome. Arthritis Rheum. 2013;65:2183–9.
Carbonella A, Mancano G, Gremese E, Alkuraya FS, Patel N, Gurrieri F, et al. An autosomal recessive DNASE1L3-related autoimmune disease with unusual clinical presentation mimicking systemic lupus erythematosus. Lupus. 2017;26:768–72.
Batu ED, Koşukcu C, Taşkıran E, Sahin S, Akman S, Sözeri B, et al. Whole exome sequencing in early-onset systemic lupus erythematosus. J Rheumatol. 2018;45:1671–9.
Bruschi M, Bonanni A, Petretto A, Vaglio A, Pratesi F, Santucci L, et al. Neutrophil extracellular traps (NETs) profiles in patients with incident SLE and lupus nephritis. J Rheumatol. 2020;47:377–86.
Ranalli M, Passarelli C, Messia V, Pardeo M, Sacco E, Insalaco A, et al. Fri0554 Dnase1l3 variant in hypocomplementemic urticarial vasculitis syndrome identifies a different clinical phenotype. Ann Rheum Dis. BMJ Publishing Group Ltd; 2019;78:972–3.
Yıldırım DG, Bakkaloğlu SA. Monogenic lupus caused by mutations in DNASE1L3: a rare cause of systemic lupus erythematosus in children. Scand J Immunol. 2022;e13162.
Kisla Ekinci RM, Balci S, Ozcan D, Atmis B, Bisgin A. Monogenic lupus due to DNASE1L3 deficiency in a pediatric patient with urticarial rash, hypocomplementemia, pulmonary hemorrhage, and immune-complex glomerulonephritis. Eur J Med Genet. 2021;64:104262.
Paç Kisaarslan A, Witzel M, Unal E, Rohlfs M, Akyildiz B, Dogan ME, et al. Refractory and fatal presentation of severe autoimmune hemolytic anemia in a child with the DNASE1L3 mutation complicated with an additional DOCK8 variant. J Pediatr Hematol Oncol. 2021;43:e452–6.
Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21.
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–30.
Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23.
Crow YJ. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr Opin Immunol. 2015;32C:7–12.
Lee-Kirsch MA. The type I interferonopathies. Annu Rev Med. 2017;68:297–315.
Lin B, Goldbach-Mansky R. Pathogenic insights from genetic causes of autoinflammatory inflammasomopathies and interferonopathies. J Allergy Clin Immunol. 2022;149:819–32.
Aicardi J, Goutières F. Systemic lupus erythematosus or Aicardi-Goutières syndrome? Neuropediatrics. 2000;31:113.
Rice GI, Forte GMA, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol. 2013;12:1159–69.
Sisirak V, Sally B, D’Agati V, Martinez-Ortiz W, Özçakar ZB, David J, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. 2016;166:88–101.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015;17:405–24.
Weill O, Decramer S, Malcus C, Kassai B, Rouvet I, Ginhoux T, et al. Familial and syndromic lupus share the same phenotype as other early-onset forms of lupus. Joint Bone Spine. 2017;84:589–93.
Galeazzi M, Morozzi G, Sebastiani GD, Bellisai F, Marcolongo R, Cervera R, et al. Anti-neutrophil cytoplasmic antibodies in 566 European patients with systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies European Concerted Action on the Immunogenetics of SLE. Clin Exp Rheumatol. 1998;16:541–6.
Rice GI, Forte G, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR a case-control study. Lancet Neurol. 2013;12:1159–69.
Rodero MP, Tesser A, Bartok E, Rice GI, Della Mina E, Depp M, et al. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nat Commun. 2017;19;8:2176.
de Jesus AA, Hou Y, Brooks S, Malle L, Biancotto A, Huang Y, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J Clin Invest. 2020;130:1669–82.
Napirei M, Ludwig S, Mezrhab J, Klöckl T, Mannherz HG. Murine serum nucleases – contrasting effects of plasmin and heparin on the activities of DNase1 and DNase1-like 3 (DNase1l3). FEBS J. 2009;276:1059–73.
Acknowledgements
We thank the patients and their parents for participating in this study. This work is dedicated to Pr Rolando Cimaz who passed away last January 29th 2022.
Funding
Y.J.C. received a state subsidy managed by the National Research Agency (France) under the “Investments for the Future” (ANR-10-IAHU-01), and the MSDAvenir fund (DEVO-DECODE Project). A.B. was supported by the National Research Agency (France) (ANR-21-CE17-0064–01).
Author information
Authors and Affiliations
Contributions
MT and AB made substantial contributions to the conception or design of the work. MT and AB drafted the manuscript. EL, CS, RP, ALM, MCM, VS, MD, AD, GN, AJ, LJ, JBG, EC, IR, DG, NF, GIR, GL, AL, PR, TW, SV, MP, YJC, TA, and AB were involved in the acquisition, analysis, or interpretation of data. All authors provided critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Leeds (East) Research Ethics Committee (reference number 10/H1307/132) and the Medical Ethics Committee of Sud Est III (Lyon, France).
Consent to Participate
Parental written informed consent was provided for inclusion of clinical data and samples in the study.
Consent for Publication
Written informed consent for publishing the results of this study considering ethical confidentiality and anonymity was obtained from the parents.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tusseau, M., Lovšin, E., Samaille, C. et al. DNASE1L3 deficiency, new phenotypes, and evidence for a transient type I IFN signaling. J Clin Immunol 42, 1310–1320 (2022). https://doi.org/10.1007/s10875-022-01287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-022-01287-5