Abstract
Purpose
Patients with heterozygous gain-of-function (GOF) mutations in STAT1 frequently exhibit chronic mucocutaneous candidiasis (CMC), immunodeficiency and autoimmune manifestations. Several treatment options including targeted therapies and hematopoietic stem cell transplantation (HSCT) are available for STAT1 GOF patients but modalities and outcomes are not well established. Herein, we aimed to unravel the effect of ruxolitinib as a bridge therapy in a patient with sporadic STAT1 T385M mutation to manage infections and other disease manifestations.
Methods
Peripheral blood mononuclear cells were isolated from the patient prior to, during ruxolitinib treatment and 6 months after HSCT. IFN-β-induced STAT1 phosphorylation/dephosphorylation levels and PMA/ionomycin-stimulated intracellular IL-17A/IFN-γ production in CD4+ T cells were evaluated. Differentially expressed genes between healthy controls and the patient prior to, during ruxolitinib treatment and post-transplantation were investigated using Nanostring nCounter Profiling Panel.
Results
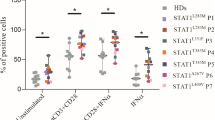
Ruxolitinib provided favorable responses by controlling candidiasis and autoimmune hemolytic anemia in the patient. Dysregulation in STAT1 phosphorylation kinetics improved with ruxolitinib treatment and was completely normalized after transplantation. TH17 deficiency persisted after ruxolitinib treatment, but normalized following HSCT. Consistent with the impairment in JAK/STAT signaling, multiple immune related pathways were found to be dysregulated in the patient. At baseline, genes related to type I IFN-related pathways, antigen processing, T-cell and B-cell functions were upregulated, while NK-cell function and cytotoxicity related genes were downregulated. Dysregulated gene expression was partially improved with ruxolitinib treatment and normalized after transplantation.
Conclusion
Our findings suggest that improved disease management and immune dysregulatory profile can be achieved with ruxolitinib treatment before transplantation and this would be beneficial to reduce the risk of adverse outcome of HSCT.



Similar content being viewed by others
References
van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365(1):54–61.
Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208(8):1635–48.
Hori T, Ohnishi H, Teramoto T, Tsubouchi K, Naiki T, Hirose Y, et al. Autosomal-dominant chronic mucocutaneous candidiasis with STAT1-mutation can be complicated with chronic active hepatitis and hypothyroidism. J Clin Immunol. 2012;32(6):1213–20.
Marodi L, Cypowyj S, Toth B, Chernyshova L, Puel A, Casanova JL. Molecular mechanisms of mucocutaneous immunity against Candida and Staphylococcus species. J Allergy Clin Immunol. 2012;130(5):1019–27.
Kagawa R, Fujiki R, Tsumura M, Sakata S, Nishimura S, Itan Y, et al. Alanine-scanning mutagenesis of human signal transducer and activator of transcription 1 to estimate loss- or gain-of-function variants. J Allergy Clin Immunol. 2016.
Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127(25):3154–64.
Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol. 2018;142(5):1665–9.
Leiding JW, Okada S, Hagin D, Abinun M, Shcherbina A, Balashov DN, et al. Hematopoietic stem cell transplantation in patients with gain-of-function signal transducer and activator of transcription 1 mutations. J Allergy Clin Immunol. 2018;141(2):704–17 e5.
Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. 2015;135(2):551–3.
Meesilpavikkai K, Dik WA, Schrijver B, Nagtzaam NMA, Posthumus-van Sluijs SJ, van Hagen PM, et al. Baricitinib treatment in a patient with a gain-of-function mutation in signal transducer and activator of transcription 1 (STAT1). J Allergy Clin Immunol. 2018;142(1):328–30 e2.
Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol. 2017;139(5):1629–40 e2.
Al Shehri T, Gilmour K, Gothe F, Loughlin S, Bibi S, Rowan AD, et al. Novel gain-of-function mutation in Stat1 sumoylation site leads to CMC/CID phenotype responsive to ruxolitinib. J Clin Immunol. 2019;39(8):776–85.
Zimmerman O, Rosler B, Zerbe CS, Rosen LB, Hsu AP, Uzel G, et al. Risks of ruxolitinib in STAT1 gain-of-function-associated severe fungal disease. Open Forum Infect Dis. 2017;4(4):ofx202.
Kiykim A, Charbonnier LM, Akcay A, Karakoc-Aydiner E, Ozen A, Ozturk G, et al. Hematopoietic stem cell transplantation in patients with heterozygous STAT1 gain-of-function mutation. J Clin Immunol. 2019;39(1):37–44.
Baris S, Alroqi F, Kiykim A, Karakoc-Aydiner E, Ogulur I, Ozen A, et al. Severe early-onset combined immunodeficiency due to heterozygous gain-of-function mutations in STAT1. J Clin Immunol. 2016;36(7):641–8.
Ramanan KM, Uppuluri R, Ravichandran N, Patel S, Swaminathan VV, Jayakumar I, et al. Successful remission induction in refractory familial hemophagocytic lymphohistiocytosis with ruxolitinib as a bridge to hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2020;67(3):e28071.
Kiykim A, Ogulur I, Dursun E, Charbonnier LM, Nain E, Cekic S, et al. Abatacept as a long-term targeted therapy for LRBA deficiency. J Allergy Clin Immunol Pract. 2019;7(8):2790–800 e15.
Baris HE, Ogulur I, Akcam B, Kiykim A, Karagoz D, Saraymen B, et al. Diagnostic modalities based on flow cytometry for chronic granulomatous disease: a multicenter study in a well-defined cohort. J Allergy Clin Immunol Pract. 2020;8(10):3525–34 e1.
Mistry P, Nakabo S, O’Neil L, Goel RR, Jiang K, Carmona-Rivera C, et al. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2019;116(50):25222–8.
Okada S, Asano T, Moriya K, Boisson-Dupuis S, Kobayashi M, Casanova JL, et al. Human STAT1 gain-of-function heterozygous mutations: chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol. 2020;40:1065–81.
Mossner R, Diering N, Bader O, Forkel S, Overbeck T, Gross U, et al. Ruxolitinib induces interleukin 17 and ameliorates chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation. Clin Infect Dis. 2016;62(7):951–3.
Bloomfield M, Kanderova V, Parackova Z, Vrabcova P, Svaton M, Fronkova E, et al. Utility of ruxolitinib in a child with chronic mucocutaneous candidiasis caused by a novel STAT1 gain-of-function mutation. J Clin Immunol. 2018;38(5):589–601.
Hammitzsch A, Chen L, de Wit J, Al-Mossawi MH, Ridley A, Sekine T, et al. Inhibiting ex-vivo Th17 responses in ankylosing spondylitis by targeting Janus kinases. Sci Rep. 2018;8(1):15645.
Kaleviste E, Saare M, Leahy TR, Bondet V, Duffy D, Mogensen TH, et al. Interferon signature in patients with STAT1 gain-of-function mutation is epigenetically determined. Eur J Immunol. 2019;49(5):790–800.
Smyth AE, Kaleviste E, Snow A, Kisand K, McMahon CJ, Cant AJ, et al. Aortic calcification in a patient with a gain-of-function STAT1 mutation. J Clin Immunol. 2018;38(4):468–70.
Vargas-Hernandez A, Mace EM, Zimmerman O, Zerbe CS, Freeman AF, Rosenzweig S, et al. Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. 2018;141(6):2142–55 e5.
Tabellini G, Vairo D, Scomodon O, Tamassia N, Ferraro RM, Patrizi O, et al. Impaired natural killer cell functions in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. 2017;140(2):553–64 e4.
Liau NPD, Laktyushin A, Lucet IS, Murphy JM, Yao S, Whitlock E, et al. The molecular basis of JAK/STAT inhibition by SOCS1. Nat Commun. 2018;9(1):1558.
Romberg N, Morbach H, Lawrence MG, Kim S, Kang I, Holland SM, et al. Gain-of-function STAT1 mutations are associated with PD-L1 overexpression and a defect in B-cell survival. J Allergy Clin Immunol. 2013;131(6):1691–3.
Moriya K, Suzuki T, Uchida N, Nakano T, Katayama S, Irie M, et al. Ruxolitinib treatment of a patient with steroid-dependent severe autoimmunity due to STAT1 gain-of-function mutation. Int J Hematol. 2020;112(2):258–62.
Loh ML, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children’s Oncology Group phase 1 consortium study (ADVL1011). Pediatr Blood Cancer. 2015;62(10):1717–24.
Acknowledgments
We thank Deniz Cansen Kahraman for the assistance in using the nCounter Nanostring analysis system.
Funding
This work was supported by the Scientific and Technological Research Council of Turkey (217S847 and 318S202) to S.B., and by National Institutes of Health grant R01 AI085090 to T.A.C.
Author information
Authors and Affiliations
Contributions
B.K., T.A.C., M.G., and S.B. conceptualized the study and wrote the manuscript. B.K., N.Y., L.M.C., and B.G. performed the experiments. N.K., A.K., S.B.E., G.O., A.O., E.K.A., and S.B. followed up the patients, provided samples, and intellectually contributed to the manuscript.
Corresponding author
Ethics declarations
The study was approved by Ethics Committee of Marmara University, School of Medicine. Written informed consents were obtained from the patients and parents.
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent to Participate
Informed consent for participation was obtained from all individuals.
Consent for Publication
Informed publication consent was obtained from all participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
The list of genes used for pathway z-score analysis. (XLSX 16 kb)
Figure S1
Representative flow cytometry plots illustrating IL-17 deficiency in the patient. IL-17A and IFN-γ production in CD4+ T cells of patient (P-Baseline) and healthy controls (HC) following stimulation with PMA (50ng/ml) and ionomycin (1 μg/ml) are shown. (PDF 105 kb)
Figure S2
Normalization of TFH percentage in the patient after ruxolitinib treatment. (A) Percentages of TFH (CXCR5+PD-1+) cells in CD4+ T cell gate in patient before (P-Baseline) and after ruxolitinib treatment (P-Ruxo) compared to healthy controls (HC) were assessed by flow cytometry. (PDF 176 kb)
Figure S3
Improvement in augmented STAT1 phosphorylation in the patient helper T cells with ruxolitinib treatment. STAT1 phosphorylation with or without IFN-γ (20ng/ml) stimulation was analyzed in patient cells before (P-Baseline) and 6 months after ruxolitinib treatment (P-Ruxo) by flow cytometry. (PDF 35 kb)
Figure S4
Restoration of STAT1 phosphorylation and dephosphorylation pattern in CD4+ T cells of the patient after HSCT. STAT1 phosphorylation was assessed in PBMCs stimulated with IFN-β for 15, 60 and 120 minutes as opposed to untreated healthy controls (HC). (PDF 514 kb)
Figure S5
Immune restoration after ruxolitinib and HSCT. (A) Comparison of IFN scores between patient, prior to (P-baseline), at 7 months of ruxolitinib therapy (P-Ruxo) and 6 months after HSCT (P-HSCT), and healthy controls. IFN score was calculated using median fold change from the values in healthy controls for the expression of 30 ISGs given in Figure 3C. (B) Comparison of antigen processing pathway z-score between patient and healthy controls. (C) Comparison of T cell function pathway z-score between patient and healthy controls. (D) Comparison of B cell function pathway z-score between patient and healthy controls. Asterisks (*) on bars show comparisons between patient and healthy controls. n.s., Not significant. **p <.01, ***p <.001 **** and p<.0001, Student unpaired 2-tailed t test for comparison between patient and healthy controls and Student paired 2-tailed t test for comparison within patient groups. (PDF 66 kb)
Figure S6
Reverse of NK cell function and cytotoxicity after ruxolitinib treatment and HSCT. (A) Comparison of NK cell function pathway z-score between patient, prior to (P-baseline), at 7 months of ruxolitinib therapy (P-Ruxo) and 6 months after HSCT (P-HSCT), and healthy controls. (B) Comparison of cytotoxicity pathway z-score between patient and healthy controls. Asterisks (*) on bars show comparisons between patient and healthy controls. n.s., Not significant. *p <.05, **p <.01, ***p <.001 **** and p<.0001, Student unpaired 2-tailed t test for comparison between patient and healthy controls and Student paired 2-tailed t test for comparison within patient groups. (PDF 34 kb)
Figure S7
Impact of ruxolitinib treatment and HSCT on SOCS1 and PD-L1 gene expression. (A) Number of probes detecting SOCS1 mRNAs in total RNA sample of patient, prior to (P-baseline), at 7 months of ruxolitinib therapy (P-Ruxo) and 6 months after HSCT (P-HSCT), and healthy controls are compared. (B) Number of probes detecting PD-L1 mRNAs in total RNA sample of patient, prior to (P-baseline), at 7 months of ruxolitinib therapy (P-Ruxo) and 6 months after HSCT (P-HSCT), and healthy controls are compared. Asterisks (*) on bars show comparisons between patient and healthy controls. n.s., Not significant. **p <.01, ***p <.001 **** and p<.0001, Student unpaired 2-tailed t test for comparison between patient and healthy controls and Student paired 2-tailed t test for comparison within patient groups. (PDF 32 kb)
Rights and permissions
About this article
Cite this article
Kayaoglu, B., Kasap, N., Yilmaz, N.S. et al. Stepwise Reversal of Immune Dysregulation Due to STAT1 Gain-of-Function Mutation Following Ruxolitinib Bridge Therapy and Transplantation. J Clin Immunol 41, 769–779 (2021). https://doi.org/10.1007/s10875-020-00943-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00943-y