Abstract
Purpose
The Primary Immune Deficiency Treatment Consortium (PIDTC) enrolled children with severe combined immunodeficiency (SCID) in a prospective natural history study of hematopoietic stem cell transplant (HSCT) outcomes over the last decade. Despite newborn screening (NBS) for SCID, infections occurred prior to HSCT. This study’s objectives were to define the types and timing of infection prior to HSCT in patients diagnosed via NBS or by family history (FH) and to understand the breadth of strategies employed at PIDTC centers for infection prevention.
Methods
We analyzed retrospective data on infections and pre-transplant management in patients with SCID diagnosed by NBS and/or FH and treated with HSCT between 2010 and 2014. PIDTC centers were surveyed in 2018 to understand their practices and protocols for pre-HSCT management.
Results
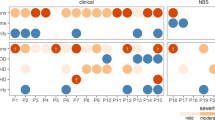
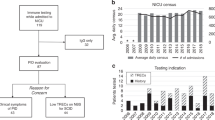
Infections were more common in patients diagnosed via NBS (55%) versus those diagnosed via FH (19%) (p = 0.012). Outpatient versus inpatient management did not impact infections (47% vs 35%, respectively; p = 0.423). There was no consensus among PIDTC survey respondents as to the best setting (inpatient vs outpatient) for pre-HSCT management. While isolation practices varied, immunoglobulin replacement and antimicrobial prophylaxis were more uniformly implemented.
Conclusion
Infants with SCID diagnosed due to FH had lower rates of infection and proceeded to HSCT more quickly than did those diagnosed via NBS. Pre-HSCT management practices were highly variable between centers, although uses of prophylaxis and immunoglobulin support were more consistent. This study demonstrates a critical need for development of evidence-based guidelines for the pre-HSCT management of infants with SCID following an abnormal NBS.
Trial Registration
NCT01186913





Similar content being viewed by others
Data Availability
Deidentified individual participant data will not be made available.
Change history
03 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10875-020-00917-0
Abbreviations
- CMV:
-
Cytomegalovirus
- EBV:
-
Epstein–Barr virus
- ERT:
-
Enzyme replacement therapy
- FH:
-
Family history
- GT:
-
Gene therapy
- HSCT:
-
Hematopoietic stem cell transplant
- MAC:
-
Myeloablative conditioning
- NBS:
-
Newborn screening
- PIDTC:
-
Primary Immunodeficiency Treatment Consortium
- PPE:
-
Personal protective equipment
- PPV:
-
Positive pressure ventilation
- SCID:
-
Severe combined immunodeficiency
- VOD:
-
Veno-occlusive disease
References
Griffith LM, Cowan MJ, Kohn DB, Notarangelo LD, Puck JM, Schultz KR, et al. Allogeneic hematopoietic cell transplantation for primary immune deficiency diseases: current status and critical needs. J Allergy Clin Immunol. 2008;122(6):1087–96.
Griffith LM, Cowan MJ, Notarangelo LD, Puck JM, Buckley RH, Candotti F, et al. Improving cellular therapy for primary immune deficiency diseases: recognition, diagnosis, and management. J Allergy Clin Immunol. 2009;124(6):1152–60.
Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–8.
Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8.
Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729–38.
Dvorak CC, Cowan MJ, Logan BR, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the primary immune deficiency treatment consortium prospective study 6901. J Clin Immunol. 2013;33(7):1156–64.
Heimall J, Logan BR, Cowan MJ, Notarangelo LD, Griffith LM, Puck JM, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130(25):2718–27.
Pai S-Y, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–46.
Haddad E, Logan BR, Griffith LM, Buckley RH, Parrott RE, Prockop SE, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood. 2018;132(17):1737–49.
Dvorak CC, Puck JM, Wahlstrom JT, Dorsey M, Melton A, Cowan MJ. Neurologic event-free survival demonstrates a benefit for SCID patients diagnosed by newborn screening. Blood Adv. 2017;1(20):1694–8.
Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117(11):3243–6.
Gaspar HB, Qasim W, Davies EG, Rao K, Amrolia PJ, Veys P. How I treat severe combined immunodeficiency. Blood. 2013;122(23):3749–58.
Kelty WJ, Beatty SA, Wu S, Hanson IC, Demmler-Harrison GJ, Martinez CA, et al. The role of breast-feeding in cytomegalovirus transmission and hematopoietic stem cell transplant outcomes in infants with severe combined immunodeficiency. J Allergy Clin Immunol Pract. 2019;7:2863–2865.e3.
Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk–acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–45.
Atkinson C, Walter S, Sharland M, Tookey P, Luck S, Peckham C, et al. Use of stored dried blood spots for retrospective diagnosis of congenital CMV. J Med Virol. 2009;81(8):1394–8.
Dergousoff BA, Vayalumkal JV, Wright NA. Survey of infection control precautions for patients with severe combined immune deficiency. J Clin Immunol. 2019;39(8):753–61.
Whitley RJ. The use of antiviral drugs during the neonatal period. Clin Perinatol. 2012;39(1):69–81.
Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–43.
Verbsky J, Thakar M, Routes J. The Wisconsin Approach to newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2012;129:622–7.
Kwan A, Church JA, Cowan MJ, et al. Newborn screening for SCID and T cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–50.
Dorsey MJ, Dvorak CC, Cowan MJ, Puck JM. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J Allergy Clin Immunol. 2017;139(3):733–42.
Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AAC, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California, 2010-2017. Pediatrics. 2019;143(2):e20182300.
Acknowledgments
Memoriam: The authors recognize the late Dr. William T. Shearer for his contribution to this and many studies of the PIDTC. We thank Catherine Chang, Tara Bani, and Elizabeth Dunn for project management and assistance.
Authorship Contributions
• Morna Dorsey, Nicola AM Wright, Natalia S. Chaimowitz, Blachy J Dávila Saldaña, Holly Miller, Michael D. Keller, Monica S. Thakar, and Ami J. Shah, MD, designed data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the final manuscript.
• Morton J Cowan, Rebecca H. Buckley, Christopher C. Dvorak, Elie Haddad, Donald B. Kohn, Luigi D. Notarangelo, Sung Yun Pai, Jennifer Puck, MD, Michael Pulsipher, and Jennifer Heimall conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript.
• Linda Griffith and Brent Logan conceptualized and designed the study, designed the data collection instruments, carried out data analyses, and reviewed and revised the manuscript.
• Rolla Abu-Arja, Jeffrey Andolina, Victor Aquino, JL Barnum, Jeffrey J. Bednarski, Monica Bhatia, Francisco A. Bonilla, Manish J. Butte, Nancy J Bunin, Sharat Chandra, Sonali Chaudhury, Karin Chen, Hey Chong, Geoff Cuvelier, MD, FRCPC, Jignesh Dalal, Magee L. DeFelice, Kenneth B. DeSantes, Lisa R Forbes, Alfred Gillio, Fred Goldman, Avni Y Joshi, Neena Kapoor, MD, Alan P. Knutsen, MD, Lisa Kobrynski, Jay A Lieberman, Jennifer W Leiding, Benjamin Oshrine, Kiran P. Patel, Susan Prockop, Troy C. Quigg, Ralph Quinones, Kirk R. Schultz, Christine Seroogy, David Shyr, Subhadra Siegel, Angela R. Smith, Troy R. Torgerson, Mark T. Vander Lugt, and Lolie C Yu collected data and critically reviewed the manuscript for important intellectual content.
• All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases (NIAID); and the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), Bethesda, MD; Public Health Service grant/cooperative agreements U54-AI082973 (PIs: MJ Cowan; September 2019 forward JM Puck and DB Kohn), U54-NS064808 and U01-TR001263 (PI: JP Krischer), R13-AI094943 (PIs: MJ Cowan; March 2018 forward JM Puck), and the Division of Intramural Research, NIAID, NIH. LD Notarangelo is supported by the Division of Intramural Research, NIAID, NIH. The PIDTC is a part of the Rare Diseases Clinical Research Network (RDCRN) of ORDR, NCATS. Collaborative work of the PIDTC with the Pediatric Blood and Marrow Transplant Consortium (PBMTC) is supported by the U54 grants above along with support of the PBMTC Operations Center by grant/cooperative agreement U10HL069254 (PI: MA Pulsipher, NHLBI/NCI) and a Johnny Cristopher Children’s Charitable Foundation St. Baldrick’s Consortium Grant. Collaborative work of the PIDTC with the Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by grant/cooperative agreement U24-CA76518 (PI: MM Horowitz) from the National Cancer Institute (NCI), NHLBI, and NIAID, NIH; and grant/cooperative agreement U01HL069294 from the NHLBI and NCI; contract HHSH250201200016C and HHSH234200637015C with the Health Resources and Services Administration (HRSA/DHHS); and grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Disclaimer
The content and opinions expressed are solely the responsibility of the authors and do not represent the official policy or position of the NIAID, ORDR, NCATS, NIH, HRSA, or any other agency of the US government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dorsey, M.J., Wright, N.A.M., Chaimowitz, N.S. et al. Infections in Infants with SCID: Isolation, Infection Screening, and Prophylaxis in PIDTC Centers. J Clin Immunol 41, 38–50 (2021). https://doi.org/10.1007/s10875-020-00865-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-020-00865-9