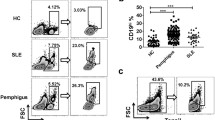
Abstract
Pemphigus vulgaris (PV) is a Th2-dominant autoimmune skin disease. We showed that indeed active PV patients had a biased Th2 response and specific IgG4 autoantibodies were dominant. To further investigate the role of antigen-specific Th2 cells in the regulation of pathogenic Dsg3-IgG antibody production, we used recombined Dsg3 protein to immunize wild-type C57BL/6 mice with aluminum hydroxide or complete Freund’s adjuvant as adjuvant. CD4+ T cells from Dsg3-immunized mice were adoptively transferred into TCR-β chain deficient mice. The transferred CD4+ T cells were readily seen in the peripheral blood and spleen, and interacted with B cells, resulting in B-cell activation. Furthermore, transferred CD4+ T cells from mice immunized with Dsg3 plus Alum with Th2 phenotype were able to render unprimed B cells to secrete Dsg3-specific IgG1 antibody in vivo. Taken together, these results provide the first demonstration of direct role of Dsg3-reactive CD4+ T (Th2) cells in the regulation of pathologic anti-Dsg3 antibody production.





Similar content being viewed by others
References
Amagai M. Pemphigus: autoimmunity to epidermal cell adhesion molecules. Advances in Dermatology. 1996;11:319–52.
Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Current Opinion in Cell Biology. 2004;16:536–43.
Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous disease due to defective epidermal cell adhesion. Advances in Immunology. 1993;53:291–325.
Hertl M, Veldman C. Pemphigus—paradigm of autoantibody-mediated autoimmunity. Skin Pharmacology and Applied Skin Physiology. 2001;14:408–18.
Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. The New England Journal of Medicine. 1982;306:1189–96.
Lin MS, Swartz SJ, Lopez A, Ding X, Fernandez-Vina MA, Stastny P, et al. Development and characterization of desmoglein-3 specific T cells from patients with pemphigus vulgaris. The Journal of Clinical Investigation. 1997;99:31–40.
Hertl M, Amagai M, Sundaram H, Stanley J, Ishii K, Katz SI. Recognition of desmoglein 3 by autoreactive T cells in pemphigus vulgaris patients and normals. The Journal of Investigative Dermatology. 1998;110:62–6.
Nishifuji K, Amagai M, Kuwana M, Toshiro I, Takeji N. Detection of antigen-specific B cells in patients with pemphigus vulgaris by enzyme-linked immunospot assay: requirement of T cell collaboration for autoantibody production. The Journal of Investigative Dermatology. 2000;114:88–94.
Takahashi H, Kuwana M, Amagai M. A single helper T cell clone is sufficient to commit polyclonal naive B cells to produce pathogenic IgG in experimental pemphigus vulgaris. Journal of Immunology. 2009;182:1740–5.
Veldman CM, Gebhard KL, Uter W, Wassmuth R, Grotzinger J, Schultz E, et al. T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. Journal of Immunology. 2004;172:3883–92.
Rizzo C, Fotino M, Zhang Y, Chow S, Spizuoco A, Sinha AA. Direct characterization of human T cells in pemphigus vulgaris reveals elevated autoantigen-specific Th2 activity in association with active disease. Clinical and Experimental Dermatology. 2005;30:535–40.
Torzecka JD, Woźniak K, Kowalewski C, Waszczykowska E, Sysa-Jedrzejowska A, Pas HH, et al. Circulating pemphigus autoantibodies in healthy relatives of pemphigus patients: coincidental phenomenon with a risk of disease development? Archives of Dermatological Research. 2007;299:239–43.
Sitaru C, Mihai S, Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Archives of Dermatological Research. 2007;299:1–8.
Bhol K, Mohimen A, Ahmed R. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatologica. 1994;189:85–9.
Green MG, Bystryn JC. Effect of intravenous immunoglobulin therapy on serum levels of IgG1 and IgG4 antidesmoglein 1 and antidesmoglein 3 antibodies in pemphigus vulgaris. Archives of Dermatology. 2008;144:1621–4.
Harman KE, Albert S, Black MM. Guidelines for the management of pemphigus vulgaris. The British Journal of Dermatology. 2003;149:926–37.
Kwon EJ, Yamagami J, Nishikawa T, Amagai M. Anti-desmoglein IgG autoantibodies in patients with pemphigus in remission. Journal of the European Academy of Dermatology and Venereology. 2008;22:1070–5.
Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. Journal of Immunology. 1997;159:2010–7.
Petersen CM, Christensen EI, Andresen BS, Moller BK. Internalization, lysosomal degradation and new synthesis of surface membrane CD4 in phorbol ester-activated T-lymphocytes and U-937 cells. Experimental Cell Research. 1992;201:160–73.
Markovic-Plese S, Pinilla C, Martin R. The initiation of the autoimmune response in multiple sclerosis. Clinical Neurology and Neurosurgery. 2004;106:218–22.
Kuwana M, Ikeda Y. The role of autoreactive T-cells in the pathogenesis of idiopathic thrombocytopenic purpura. International Journal of Hematology. 2005;81:106–12.
Veldman C, Eming R, Wolff-Franke S, Sonderstrup G, Kwok WW, Hertl M. Detection of low avidity desmoglein 3-reactive T cells in pemphigus vulgaris using HLA-DR beta 1*0402 tetramers. Clinical Immunology. 2007;122:330–7.
Veldman C, Stauber A, Wassmuth R, Uter W, Schuler G, Hertl M. Dichotomy of autoreactive Th1 and Th2 cell responses to desmoglein 3 in patients with pemphigus vulgaris (PV) and healthy carriers of PV-associated HLA class II alleles. Journal of Immunology. 2003;170:635–42.
Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annual Review of Immunology. 1990;8:303–33.
Kotowicz K, Callard RE. Human immunoglobulin class and IgG subclass regulation: dual action of interleukin-4. European Journal of Immunology. 1993;23:2250–6.
Pan M, Zhou Y. WP Li, J. Zheng. Modulation of desmoglein-3-specific T cell response and pathogenic antibody generation in mice. Immunology Letters. 2008;120:6–13.
Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clinical and Experimental Allergy. 2009;39:469–77.
Anzai H, Fujii Y, Nishifuji K, Aoki-Ota M, Ota T, Amagai M, et al. Conformational epitope mapping of antibodies against desmoglein 3 in experimental murine pemphigus vulgaris. Journal of Dermatological Science. 2004;35:133–42.
Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. Journal of Immunology. 2001;167:5439–48.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30771932, 81171499). We thank the staff of Shanghai Institute of Immunology, Institute of Medical Science, School of Medicine, Shanghai Jiao Tong University, for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, H., Chen, Y., Zhou, Y. et al. Cognate Th2–B Cell Interaction is Essential for the Autoantibody Production in Pemphigus Vulgaris. J Clin Immunol 32, 114–123 (2012). https://doi.org/10.1007/s10875-011-9597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9597-4