Abstract
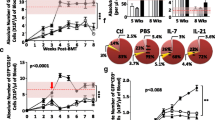
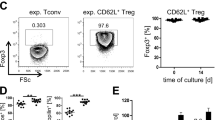
Using the C57BL/6→(C57BL/6 x DBA/2)F1-hybrid model of acute graft-versus-host disease (GVHD), we previously showed that treating the donor mice with palifermin provides protection from morbidity and a shift from Th1 to Th2 cytokine production. To determine whether thymic stromal lymphopoietin (TSLP) is involved in palifermin-mediated immune modulation, we used donors from the following groups: (1) untreated wild-type donors, (2) palifermin-treated wild-type donors, (3) untreated TSLPR−/− donors, and (4) palifermin-treated TSLPR−/− donors. Survival in the recipients was 0%, 100%, 31%, and 0%, for groups 1–4, respectively, indicating that TSLP responsiveness is required for palifermin-mediated protection from GVHD. We also found that the increases in Th2 cytokine levels that are induced by palifermin treatment are obviated in TSLPR−/− donors, and that protection from GVHD (group 2) is associated with a higher percentage of CD4+CD25+Foxp3+ cells in the graft. Collectively, our findings show that when palifermin and TSLP act in concert, the predominant effect is protection in this model.




Similar content being viewed by others
References
Woodruff JM, Eltringham JR, Casey HW. Early secondary disease in the Rhesus monkey. I. A comparative histopathologic study. Lab Invest. 1969;20:499–511.
Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175:405–13.
Stuber E, Buschenfeld A, von Freier A, Arendt T, Folsch UR. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL–Fas interaction: effect of pentoxifylline on the development of mucosal atrophy. Gut. 1999;45:229–35.
Garside P, Hutton AK, Severn A, Liew FY, Mowat AM. Nitric oxide mediates intestinal pathology in graft-vs.-host disease. Eur J Immunol. 1992;22:2141–5.
Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–5.
Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–6.
Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–5.
Yi ES, Shabaik AS, Lacey DL, Bedoya AA, Yin S, Housley RM, et al. Keratinocyte growth factor causes proliferation of urothelium in vivo. J Urol. 1995;154:1566–70.
Staiano-Coico L, Krueger JG, Rubin JS, D’Limi S, Vallat VP, Valentino L, et al. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993;178:865–78.
Bottaro DP, Rubin JS, Ron D, Finch PW, Florio C, Aaronson SA. Characterization of the receptor for keratinocyte growth factor. Evidence for multiple fibroblast growth factor receptors. J Biol Chem. 1990;265:12767–70.
Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, et al. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–77.
Ulich TR, Yi ES, Cardiff R, Yin S, Bikhazi N, Biltz R, et al. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994;144:862–8.
Parrott JA, Kim G, Mosher R, Skinner MK. Expression and action of keratinocyte growth factor (KGF) in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2000;167:77–87.
Pierce GF, Yanagihara D, Klopchin K, Danilenko DM, Hsu E, Kenney WC, et al. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994;179:831–40.
Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993;92:969–77.
Yi ES, Williams ST, Lee H, Malicki DM, Chin EM, Yin S, et al. Keratinocyte growth factor ameliorates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol. 1996;149:1963–70.
Ulich TR, Whitcomb L, Tang W, O’Conner Tressel P, Tarpley J, Yi ES, et al. Keratinocyte growth factor ameliorates cyclophosphamide-induced ulcerative hemorrhagic cystitis. Cancer Res. 1997;57:472–5.
Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–9.
Frank S, Munz B, Werner S. The human homologue of a bovine non-selenium glutathione peroxidase is a novel keratinocyte growth factor-regulated gene. Oncogene. 1997;14:915–21.
Takeoka M, Ward WF, Pollack H, Kamp DW, Panos RJ. KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am J Physiol. 1997;272:L1174–80.
Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595–601.
Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR. Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood. 1998;92:3960–7.
Krijanovski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS, et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94:825–31.
Ellison CA, Natuik SA, Fischer JM, McIntosh AR, Scully SA, Bow EJ, et al. Effect of recombinant human keratinocyte growth factor (rHuKGF) on the immunopathogenesis of intestinal graft-vs.-host disease induced without a preconditioning regimen. J Clin Immunol. 2004;24:197–211.
Panoskaltsis-Mortari A, Taylor PA, Rubin JS, Uren A, Welniak LA, Murphy WJ, et al. Keratinocyte growth factor facilitates alloengraftment and ameliorates graft-versus-host disease in mice by a mechanism independent of repair of conditioning-induced tissue injury. Blood. 2000;96:4350–6.
Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–91.
Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–600.
Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–60.
Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–37.
Ellison CA, Makar BM, Wiseman JM, Gheorghiu I, Taniguchi M, Gartner JG. Palifermin mediates immunoregulatory effects in addition to its cytoprotective effects in mice with acute graft-versus-host disease. J Clin Immunol. 2008;28:600–15.
Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, et al. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–78.
Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43.
Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64.
Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–70.
Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–8.
Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404.
Shi L, Leu SW, Xu F, Zhou X, Yin H, Cai L, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129:202–10.
Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008;181:4311–9.
Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–14.
Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008;181:6557–62.
Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–68.
Gartner JG, Merry AC, Smith CI. An analysis of pulmonary natural killer cell activity in F1-hybrid mice with acute graft-versus-host reactions. Transplantation. 1988;46:879–86.
Ellison CA, Fischer JM, HayGlass KT, Gartner JG. Murine graft-versus-host disease in an F1-hybrid model using IFN-gamma gene knockout donors. J Immunol. 1998;161:631–40.
Bruinsma M, van Soest PL, Leenen PJ, Lambrecht BN, Cupedo T, Lowenberg B, et al. Keratinocyte growth factor induces expansion of murine peripheral CD4+Foxp3+ regulatory T cells and increases their thymic output. J Immunol. 2007;179:7424–30.
Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–11.
Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9.
Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6.
Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12.
Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99.
Ellison CA, Natuik SA, McIntosh AR, Scully SA, Danilenko DM, Gartner JG. The role of interferon-gamma, nitric oxide and lipopolysaccharide in intestinal graft-versus-host disease developing in F1-hybrid mice. Immunology. 2003;109:440–9.
Acknowledgments
We are grateful to Mr. Monroe Chan for sharing his expertise and assisting with the flow cytometry analyses. This project is supported by an operating grant to JGG, CAE and K.T. HayGlass from the Canadian Institute of Health Research (MOP 67065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellison, C.A., Lissitsyn, Y.V., Packiasamy, J.A. et al. Role of Thymic Stromal Lymphopoietin (TSLP) in Palifermin-Mediated Immune Modulation and Protection from Acute Murine Graft-Versus-Host Disease. J Clin Immunol 31, 406–413 (2011). https://doi.org/10.1007/s10875-010-9491-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-010-9491-5