Abstract
Introduction
The high mobility group box 1 protein (HMGB-1)/advanced glycation end products (RAGE) system is recently shown to play an important part in immune/inflammatory disorders. However, the association of this system in systemic sclerosis (SSc) remains unknown.
Materials and Methods
To determine clinical association of serum levels of HMGB-1 and soluble RAGE (sRAGE) in patients with SSc, sera from 70 patients with SSc and 25 healthy controls were examined by enzyme-linked immunosorbent assay. Sera from tight-skin mice and bleomycin-induced scleroderma mice, animal models for SSc, were also examined. Skin HMGB-1 and RAGE expression was assessed by immunohistochemistry.
Results and Discussion
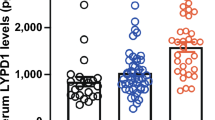
Serum HMGB-1 and sRAGE levels in SSc were higher than those in controls. Similarly, HMGB-1 and sRAGE levels in animal SSc models were higher than those in control mice. SSc patients with elevated HMGB-1 and sRAGE levels had more frequent involvement of several organs and immunological abnormalities compared to those with normal levels. Furthermore, HMGB-1 and sRAGE levels correlated positively with modified Rodnan total skin thickness score and negatively with pulmonary function test.
Conclusions
HMGB-1 and sRAGE expression in the sclerotic skin was more intense than normal skin. These results suggest that elevated serum HMGB-1 and sRAGE levels are associated with the disease severity and immunological abnormalities in SSc.





Similar content being viewed by others
References
Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003;120:542–7. doi:10.1046/j.1523-1747.2003.12097.x.
La Montagna G, D’Angelo S, Valentini G. Cross-sectional evaluation of ykl-40 serum concentrations in patients with systemic sclerosis. Relationship with clinical and serological aspects of disease. J Rheumatol. 2003;30:2147–51.
Szegedi A, Czirjak L, Unkeless JC, Boros P. Serum cytokine and anti-fc gamma r autoantibody measurements in patients with systemic sclerosis. Acta Derm Venereol. 1996;76:21–3.
Arnett FC. Is scleroderma an autoantibody mediated disease? Curr Opin Rheumatol. 2006;18:579–81. doi:10.1097/01.bor.0000245726.33006.c3.
Bell CW, Jiang W, Reich CF 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–25. doi:10.1152/ajpcell.00616.2005.
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi:10.1038/sj.embor.7400205.
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–7. doi:10.1016/j.it.2005.04.009.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. Hmg-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi:10.1126/science.285.5425.248.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi:10.1038/nri1594.
Bartling B, Fuchs C, Silber RE, Simm A. Fibroblasts mediate induction of high mobility group box protein 1 in lung epithelial cancer cells by diffusible factors. Int J Mol Med. 2007;20:217–24.
Ren D, Sun R, Wang S. Role of inducible nitric oxide synthase expressed by alveolar macrophages in high mobility group box 1-induced acute lung injury. Inflamm Res. 2006;55:207–15. doi:10.1007/s00011-006-0072-2.
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and rage. Nat Immunol. 2007;8:487–96. doi:10.1038/ni1457.
Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (rage) directly binds to ERK by a d-domain-like docking site. FEBS Lett. 2003;550:107–13. doi:10.1016/S0014-5793(03)00846-9.
Malherbe P, Richards JG, Gaillard H, Thompson A, Diener C, Schuler A, et al. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res. 1999;71:159–70. doi:10.1016/S0169-328X(99)00174-6.
Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–109. doi:10.1042/BJ20021371.
Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH, et al. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (rage) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol. 2004;40:1203–11. doi:10.1016/j.molimm.2003.11.027.
Nawroth PP, Stern DM. Soluble forms of rage: an index of vascular stress? A commentary on “Soluble rage in type 2 diabetes: association with oxidative stress”. Free Radic Biol Med. 2007;43:506–10. doi:10.1016/j.freeradbiomed.2007.04.014.
Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–6. doi:10.1164/rccm.200402-188OC.
Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, et al. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975–9.
Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–65. doi:10.1152/ajplung.00359.2004.
Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Synovial fluid expression of autoantibodies specific for rage relates to less erosive course of rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1367–71. doi:10.1093/rheumatology/kem141.
Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7:R817–24. doi:10.1186/ar1749.
Bopp C, Hofer S, Weitz J, Bierhaus A, Nawroth PP, Martin E, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res 2008;147:79–83.
Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I: local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112:456–62. doi:10.1046/j.1523-1747.1999.00528.x.
Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82:493–512.
Committee SfSCotARADaTC. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. doi:10.1002/art.1780230510.
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5.
Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobeservar variability in 3 independent studies. J Rheumatol. 1993;20:1892–6.
Sato S, Ihn H, Kikuchi K, Takehara K. Antihistone antibodies in systemic sclerosis: association with pulmonary fibrosis. Arthritis Rheum. 1994;37:391–4. doi:10.1002/art.1780370313.
Nishijima C, Sato S, Hasegawa M, Nagaoka T, Hirata A, Komatsu K, et al. Renal vascular damage in Japanese patients with systemic sclerosis. Rheumatology. 2001;40:406–109. doi:10.1093/rheumatology/40.4.406.
Kokkola R, Li J, Sundberg E, Aveberger AC, Palmblad K, Yang H, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–8. doi:10.1002/art.11161.
Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10:445–8.
Sakurai S, Yamamoto Y, Tamei H, Matsuki H, Obata K, Hui L, et al. Development of an ELISA for esRAGE and its application to type 1 diabetic patients. Diabetes Res Clin Pract. 2006;73:158–65. doi:10.1016/j.diabres.2005.12.013.
Alecu M, Geleriu L, Coman G, Galatescu L. The interleukin-1, interleukin-2, interleukin-6 and tumour necrosis factor alpha serological levels in localised and systemic sclerosis. Rom J Intern Med. 1998;36:251–9.
Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333–44. doi:10.1007/s00403-005-0635-z.
Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. Rage mediates a novel proinflammatory axis: a central cell surface receptor for s100/calgranulin polypeptides. Cell. 1999;97:889–901. doi:10.1016/S0092-8674(00)80801-6.
Li J, Schmidt AM. Characterization and functional analysis of the promoter of rage, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–506. doi:10.1074/jbc.272.26.16498.
Yamagishi S, Adachi H, Nakamura K, Matsui T, Jinnouchi Y, Takenaka K, et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism. 2006;55:1227–31. doi:10.1016/j.metabol.2006.05.007.
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2003;279:7370–7. doi:10.1074/jbc.M306793200.
Lin KI, Kao YY, Kuo HK, Yang WB, Chou A, Lin HH, et al. Reishi polysaccharides induce immunoglobulin production through the tlr4/tlr2-mediated induction of transcription factor blimp-1. J Biol Chem. 2006;281:24111–23. doi:10.1074/jbc.M601106200.
Ek M, Popovic K, Harris HE, Naucler CS, Wahren-Herlenius M. Increased extracellular levels of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in minor salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 2006;54:2289–94. doi:10.1002/art.21969.
Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, et al. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–45. doi:10.1002/art.21398.
Bruchfeld A, Qureshi AR, Lindholm B, Barany P, Yang L, Stenvinkel P, et al. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med. 2008;14:1–15. doi:10.2119/2007-00107.
van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products (rage) in HMGB1 induced inflammation in vivo. Shock. 2008. PMID: 18665043.
Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–73. doi:10.1097/01.CCM.0000155991.88802.4D.
Sundberg E, Grundtman C, Af Klint E, Lindberg J, Ernestam S, Ulfgren AK, et al. Systemic tnf blockade does not modulate synovial expression of the pro-inflammatory mediator hmgb1 in rheumatoid arthritis patients—a prospective clinical study. Arthritis Res Ther. 2008;10:R33. doi:10.1186/ar2387.
Kielty CM, Raghunath M, Siracusa LD, Sherratt MJ, Peters R, Shuttleworth CA, et al. The tight skin mouse: demonstration of mutant fibrillin-1 production and assembly into abnormal microfibrils. J Cell Biol. 1998;140:1159–66. doi:10.1083/jcb.140.5.1159.
Bona C, Rothfield N. Autoantibodies in scleroderma and tightskin mice. Curr Opin Immunol. 1994;6:931–7. doi:10.1016/0952-7915(94)90016-7.
Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006;125:661–9. doi:10.1007/s00418-005-0116-7.
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of rage-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi:10.1038/35012626.
Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, et al. The pattern recognition receptor (rage) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–15. doi:10.1084/jem.20030800.
Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, et al. Serum levels of srage, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol Med. 2007;13:1–9. doi:10.2119/2006-00090.
Ogawa F, Shimizu K, Muroi E, Hara T, Hasegawa M, Takehara K, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology. 2006;45:815–8. doi:10.1093/rheumatology/kel012.
Simonini G, Pignone A, Generini S, Falcini F, Cerinic MM. Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology. 2000;155:1–15. doi:10.1016/S0300-483X(00)00272-9.
Acknowledgment
We thank Ms. Y. Yamada, M. Yozaki, A. Usui, and K. Shimoda for technical assistance. This work was supported by a grant of Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (to S. Sato).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshizaki, A., Komura, K., Iwata, Y. et al. Clinical Significance of Serum HMGB-1 and sRAGE Levels in Systemic Sclerosis: Association with Disease Severity. J Clin Immunol 29, 180–189 (2009). https://doi.org/10.1007/s10875-008-9252-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9252-x