Abstract
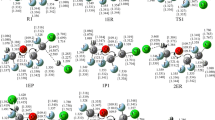
The multi-channel reactions (1) CCl3CH2OH+Cl→ products and (2) CCl3CH2OH+OH→ products have been investigated by using the dual-level direct dynamics method. Two reaction channels, i.e., methylene- and hydroxyl-hydrogen abstraction, are identified for each reaction. The optimized geometries and frequencies of the stationary points are calculated at the B3LYP/6-311G(d,p) and MP2/6-311G(d,p) levels. Higher-level energies are obtained at the MC-QCISD and G3(MP2) levels based on the B3LYP and MP2 geometries, respectively, as well as by the CCSD(T)/6-31G(d)+CF method using the B3LYP geometries. Complexes with energies lower than those of the reactants are located at the entrance of each reaction channel. The rate constants for each reaction channel are evaluated by using the canonical variational transition state theory (CVT) incorporating the small-curvature tunneling (SCT) correction in a temperature range of 200–2000 K at the MC-QCISD//B3LYP/6-311G(d,p) level. The agreement of the calculated rate constants and experimental values for two reactions is seen to be remarkably good. Theoretical results indicate that in a low temperature range, the branching ratio to the hydroxyl-H-abstraction channel for both reactions is found negligible. The reactions proceed practically via methylene-H-abstraction yielding the products of CCl3CHOH+HCl and CCl3CHOH+H2O, respectively; while for reaction of CCl3CH2OH+Cl, hydroxyl-H-abstraction channel appears to be probable with the increase of temperature. The enthalpies of formation for the CCl3CH2OH, CCl3CHOH, and CCl3CH2O species are evaluated via isodesmic reactions at several levels.




Similar content being viewed by others
References
Becke, A.D.: J. Chem. Phys. 98, 1372 (1993)
Chase Jr., M.W.: NIST-JANAF Themochemical Tables, Fourth Edition. J. Phys. Chem. Ref. Data. 9, 1–1951 (1998)
Chuang, Y.Y., Corchado, J.C., Truhlar, D.G.: J. Phys. Chem. 103, 1140 (1999)
Corchado, J.C., Chuang, Y.-Y., Fast, P.L., Hu, W.-P., Liu, Y.-P., Lynch, G.C., Nguyen, K.A., Jackels, C.F., Fernandez-Ramos, A., Ellingson, B.A., Lynch, B.J., Zheng, J.J., Melissas, V.S., Villa, J., Rossi, I., Coitino, E.L., Pu, J.Z., Albu, T.V., Steckler, R., Garrett, B.C., Isaacson, A.D., Truhlar, D.G.: POLYRATE, version 9.7, University of Minnesota, Minneapolis (2007)
Curtiss, L.A., Redfem, P.C., Raghavachari, K., Rassolov, V., Pople, J.A.: J. Chem. Phys. 110, 4703 (1999)
DeMore, W.B., Sander, S.P., Golden, S.P., Howard, C.J., Golden, D.M., Kolb, C.E., Hampson, R.F., Molina, M.J.: Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling (1997)
Distelrath, V., Boesl, U.: Faraday Discuss. Chem. Soc. 115, 161 (2000)
Fast, P.L., Truhlar, D.G.: J. Phys. Chem. 104, 6111 (2000)
Fernández-Ramos, A., Ellingson, B.A., Meana-Pañeda, R., Marques, J.M.C., Truhlar, D.G.: Theor. Chem. Acc. 118, 813 (2007)
Fisher, D.A., Hales, C.H., Wang, W.C., Ko, M.K.W., Sze, N.D.: Nature (London) 344, 513 (1990)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, N.J., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford (2009)
Garrett, B.C., Truhlar, D.G.: J. Chem. Phys. 70, 1593 (1979a)
Garrett, B.C., Truhlar, D.G.: J. Am. Chem. Soc. 101, 4534 (1979b)
Garrett, B.C., Truhlar, D.G.: J. Chem. Phys. 76, 1853 (1982)
Garrett, B.C., Truhlar, D.G., Grev, R.S., Magnuson, A.W.: J. Phys. Chem. 84, 1730 (1980)
Gonzalez, C., Schlegel, H.B.: J. Phys. Chem. 94, 5523 (1990)
Hammitt, J.K., Camm, F., Connell, P.S., Mooz, W.E., Wolf, K.A., Wuebbles, D.J., Bamezoi, A.: Nature (London) 330, 711 (1987)
Hanel, R.A.: J. Geophys. Res. 77, 2629 (1972)
Houghton, J.T., et al. (eds.) WMO/UNEP, Climate Change 1995, The IPCC Scientific Assessment. Cambridge University Press: Cambridge (1996)
Hu, W.P., Truhlar, D.G.: J. Am. Chem. Soc. 118, 860 (1996)
Kaiser, E.W.: Int. J. Chem. Kinet. 25, 667 (1993)
Kuchitsu, K.: Structure of Free Polyatomic Molecules Basic Data 1, 35 (1998)
Lee, C., Yang, W., Parr, R.G.: Phys. Rev. B 37, 785 (1998)
Lei, W.F., Zhang, R.Y., McGivern, W.S., Derecskei-Kovacs, A., North, S.W.: Chem. Phys. Lett. 326, 109 (2000)
Liu, Y.-P., Lynch, G.C., Truong, Y.N., Lu, D., Truhlar, D.G., Garrett, B.C.: J. Am. Chem. Soc. 115, 2408 (1993)
Lu, D.H., Truong, T.N., Melissas, V.S., et al.: Comput. Phys. Commun. 71, 235 (1992)
Molina, M.J., Rowland, F.S.: Nature (London) 249, 810 (1974)
Platz, J., Nielsen, O.J., Sehested, J., Wallington, T.J.: J. Phys. Chem. 99, 570 (1995)
Rebbert, R.E., Ausloos, P.J.: J. Photochem. 4, 419 (1975)
Sehested, J.: Int. J. Chem. Kinet. 26, 1023 (1994)
Shimanouchi, T.: Tables of molecular vibrational frequencies consolidated volume I, National Bureau of Standards. 1–160 (1972)
Suh, I., Lei, W.F., Zhang, R.Y.: J. Phys. Chem. 105, 6471 (2001)
Truhlar, D.G. In: Heidrich, D. (d.) The Reaction Path in Chemistry: Current Approaches and Perspectives, pp. 229. Kluwer, Dordrecht, The Netherlands (1995)
Truhlar, D.G., Garrett, B.C.: Acc. Chem. Res. 13, 440 (1980)
Truhlar, D.G., Garrett, B.C.: Annu. Rev. Phys. Chem. 35, 159 (1984)
Truhlar, D.G., Isaacson, A.D., Garrett, B.C. In: Baer, M. (ed.) The Theory of Chemical Reaction Dynamics, pp. 65. CRC Press, Boca Raton, FL (1985)
Truhlar, D.G., Garrent, B.C., Klippenstein, S.J.: J. Phys. Chem. 100, 12771 (1996)
Wadso, I.: Acta Chem. Scand. 20, 544 (1966)
Wallington, T.J., Dagaut, P., Kurylo, M.J.: J. Phys. Chem. 92, 5024 (1988)
WMO/UNEP, Atmospheric Ozone: World Meterological Organization Global Ozone Research and Monitoring Project-Report No.16 (1985). World Meteorological Organization: Geneva, Switzerland (1986)
Young, D.C.: Computational Chemistry: A practical Guide for Applying Techniques to Real-World Problems, p. 227. Wiley, New York (2001)
Zhang, D., Zhang, R.Y., Park, J., North, S.W.: J. Am. Chem. Soc. 124, 9600 (2002)
Acknowledgment
We thank Professor Donald G. Truhlar for providing of the POLYRATE 9.7 program. This work was supported by the National Natural Science Foundation of China (21003036), the Science Foundation of Henan Province (2008A150005), Science Foundation of Henan University (2009YBZR013, SBGJ090507), Doctor Foundation of Henan University.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Table S1
(DOC 71 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Liu, S. & Zhang, Jl. Reactions of chlorine atoms and hydroxyl radicals with trichloroethanol: a mechanistic and kinetic study. J Atmos Chem 65, 73–87 (2010). https://doi.org/10.1007/s10874-011-9182-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-011-9182-5