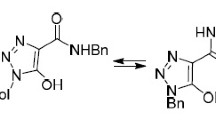
Abstract
Structures are described for the aldehyde 3,5-bis[2-(2′-formylphenoxymethyl)]-1,2,4-triazole and the new pyrazole compound 3,5-bis[(3′,5′-dimethyl)methylpyrazol-1′N-yl)]-1,2,4-triazole, for which the synthesis is given. The former crystallises in the space group Pna21 and the latter in P1[bar]. The remaining triazole N–H moiety forms hydrogen bonds, and is not disordered in the solid state. This latter property and molecular twisting features associated with the molecules' individually chiral conformations are commented upon. The electronic spectra are reported, with DFT calculations indicating that the much longer-wavelength UV absorptions of the aldehyde are dominated by the benzaldehyde moieties.
Graphical Abstract
A dialdehyde and a bis(pyrazole) derivative of 1,2,4-triazole are elucidated. The pyrazole derivative displays triazole-based H-bonding in the solid state which links molecules of individually opposite chirality into centrosymmetric dimers.













Similar content being viewed by others
References
Bradshaw JS, Krakowiak KE, Huszthy P, Izatt RM (1991) J Heterocycl Chem 28:773
Bradshaw JS, Jones BA, Nielsen RB, Spencer NO, Thompson PK (1983) J Heterocycl Chem 20:957
Bradshaw JS, Chamberlin DA, Harrison PE, Wilson BE, Arena G, Dalley NK, Lamb JD, Izatt RM, Morin FG, Grant DM (1985) J Org Chem 50:3065
Bradshaw JS, Nielsen RB, Tse PK, Arena G, Wilson BE, Dalley NK, Lamb JD, Christensen JJ, Izatt RM (1986) J Heterocycl Chem 23:361
Bradshaw JS, McDaniel CW, Skidmore BD, Nielsen RB, Wilson BE, Dalley NK, Izatt RM (1987) J Heterocycl Chem 24:1085
Alonso JM, Martin MR, Mendoza JD, Torres T, Elguerro J (1988) Heterocycles 26:989
Fernandez-Lazaro F, Mendoza JD, Mo O, Rodriguez-Morgade S, Torres T, Yanez M (1989) J Chem Soc Perkin Trans II:797
Mendoza JD, Ontaria JM, Ortega MC, Torres T (1992) Synthesis 398
Duro JA, Ontaria JM, Sastre A, Schafer W, Torres T (1993) J Chem Soc Dalton Trans 2595
Martinez-Diaz MV, Mendoza JD, Torres T (1994) Tetrahedron Lett 35:7669
Cabezon B, Irurzun M, Torres T, Vasquez P (1998) Tetrhedron Lett 39:1067
Nicolau M, Esperanza S, Torres T (2002) J Org Chem 67:1392
Tarrago G, Marzin C, Najimi O, Pellegrin V (1990) J Org Chem 55:420
Elwahy AHM, Abbas AA (2000) Tetrahedron 56:885
Elwahy AHM, Abbas AA, Kassab RM (2002) Synthesis 2:260
Alcaide E, Ayala C, Dinares I, Mesquida N (2001) J Org Chem 66:2291
Zhang HZ, Damu GLV, Cai GX, Zhou CH (2014) Curr Org Chem 18:359–406
Wu L, He P, Li Z, Wang Q, Yang J, Sinditskii VP, Zhang JG (2019) New J Chem 43:4975–4979
Brotschi C, Bolli MH, Gatfield J, Heidmann B, Jenck F, Roch C, Sifferlen T, Treiber A, Williams JT, Boss C (2020) ChemMedChem 15:430–448
Izatt RM, LindH GC, Bruening RL, Huszthy P, McDaniel CW, Bradshaw JS, Christensen JJ (1988) Anal Chem 60:1694
Elshani S, Apgar P, Wang S, Wai CM (1994) J Heterocycl Chem 31:1271
Elshani S, Wai CM, Shreeve JM, Rogers RD, Bartsch RA (2005) J Heterocycl Chem 42:621
Nozari M, Addison AW, Reeves GT, Zeller M, Jasinski JP, Kaur M, Gilbert JG, Hamilton CR, Popovitch J, Wolf LM, Crist LE, Bastida N (2018) J Heterocyc Chem 55:1291
Parsons S, Flack HD, Wagner T (2013) Acta Cryst B69:249–259
Sheldrick GM (2015) Acta Cryst A71:3–8
Sheldrick GM (2015) Acta Cryst C71:3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339–341
Adamek M (1960) Coll Czech Chem Commun 25:1964
Novikov SS, Brusnikinia VM, Rudenko VA (1969) Khim Geterotsikl Soedin 5:157
Beitelman AD, Sieracki NA, Zeller M, Ferrence GM (2007) Acta Cryst E63:o2739
Jeffrey GA, Ruble JR, Yates JH (1983) Acta Cryst B39:388
Acknowledgements
JPJ acknowledges the NSF-MRI program (Grant No.CHE-1039027) for funding of the Gemini X-ray diffractometer. We thank Drexel University (SE and AWA) and Stockton University (GTR) for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elshani, S., Jasinski, J.P., Addison, A.W. et al. Two Bifunctionalised Derivatives of 1,2,4-Triazole. J Chem Crystallogr 52, 458–468 (2022). https://doi.org/10.1007/s10870-021-00916-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00916-y