Abstract
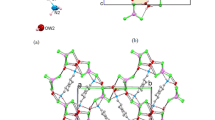
A new interhalogen ionic liquid [C17H25N3Br]+[IBr2]− (IL-1) was synthesized from its triazolium iodide precursor [C17H26N3+I−] in THF under cold and basic conditions. X-ray diffraction data showed that IL-1 is composed of two moieties contained in a triclinic unit cell; the five-membered triazolium cation and an almost linear iodidobromide anion, IBr2−. The Br atoms of the anion form quadfurcated C–H···Br hydrogen bonds with neighbouring cationic halo-1,2,3-triazolium H-species. Intermolecular Br···Br halogen bonds and I···πtriazole interactions form a distinctive ring-like pattern that links together four molecular units in the crystal packing. Hirshfeld surface analysis revealed that the most significant dnorm surface contribution at 59% is due to H···H and reciprocal C···H contacts, while the Br···Br contacts only contributed 3%. The prevalence of extensive H···H and C···H contacts is potentially due to the linear aliphatic chain, the N-octyl wingtip substituent of the triazolyl moiety. The Hirshfeld surface mapping also shows the contribution of intermolecular C–H···Br interactions at 26% of all contacts. The title compound (IL-1) showed interesting photophysical properties in MeCN solution, with an absorption band at 254 nm and two-shoulder emission bands due to strong π → π∗ transitions from the triazolium moiety, indicating the presence of two energetically associated species.
Graphical Abstract
Structural and photophysical studies on a new ionic liquid compound containing a mixed halide anion [C17H25N3Br]+[IBr2]− have yielded important results on intermolecular interactions between the triazolium cation and the iodidobromide counterion.








Similar content being viewed by others
References
Svensson PH, Kloo L (2003) Chem Rev 103:1649–1684
Burgenmeister B, Sonnenberg K, Riedel S, Krossing I (2017) Chem Eur J 23:11312
Mooney RCL, Kristallogr Z (1938) Kristallgeom Kristallphys Kristallchem 98:377
Buckles RE, Mills JF (1954) J Am Chem Soc 76:3716
Naito T, Tateno A, Udagawa T, Kobayashi H, Kato R, Kobayashi A, Nogami T (1994) J Chem Soc Faraday Trans 90:763
Svensson PH, Kloo LJ (2000) Chem Soc Dalton Trans 2449:22–26
Gorlov M, Pettersson H, Hagfeldt A, Kloo L (2007) Inorg Chem 46:3566
Bortolini O, Bottai M, Chiappe C, Conte V, Pieraccini D (2002) Green Chem 4:621
Bagno A, Butts C, Chiappe C, D’Amico F, Lord JCD, Pieraccini D, Rastrelli F (2005) Org Biomol Chem 3:1624
Van den Bossche A, De Witte E, Dehaen W, Binnemans K (2018) Green Chem 20:3327
Deshmukh A, Gore B, Thulasiram HV, Swamy VP (2015) RSC Adv 5:88311–88315
Mncube SG, Bala MD (2016) J Mol Liq 215:396–401
Muzart J (2006) Adv Synth Catal 348:275–295
Bruker (2009) APEXII. Bruker AXS Inc, Madison
Bruker (2009) SAINT. Bruker AXS Inc, Madison
Bruker (2009) SADABS. Bruker AXS Inc, Madison
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Sheldrick GM (2015) Acta Crystallogr C 71:3–8
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) J Appl Cryst 53:226–235
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J. Appl. Cryst. 42:339–341
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. University of Western Australia
Hirshfeld FL (1977) Theor Chim Acta 44:129–138
Spackman MA, Jayatilaka D (2009) CrystEngComm 11:19–32
Spackman MA, McKinnon JJ (2002) CrystEngComm 4:378–392
Seth SK, Saha NC, Ghosh S, Kara T (2011) Chem Phys Lett 506:309–314
Tepper R, Schulze B, Jäger M, Friebe C, Scharf DH, Görls H, Schubert US (2015) Org Chem 80(6):3139–3150
Mercurio JM, Knighton RC, Cookson J, Beer PD (2014) Eur J Chem 20:11740–11749
Giese M, Albrecht M, Bohnen C, Repenko T, Valkonen A, Rissanen K (2014) Dalton Trans 43:1873–1880
Buist AR, Kennedy AR (2014) Cryst Growth Des 14:6508–6513
Maleckis A, Kampf JW, Sanford MS (2013) J Am Chem Soc 135:6618–6625
Chernov’yants MS, Burykin IV, Kostrub VV, Tsupak EB, Starikova ZA, Kirsanova JA (2012) J. Mol. Struct. 110:98–103
d’Agostino S, Braga D, Grepioni F, Taddei P (2014) Cryst Growth Des 14(2):821–829
Giese M, Albrecht M, Ivanova G, Valkonen A, Rissanen K (2012) Supramol Chem 24:48–55
Cristiani F, Demartin F, Devillanova FA, Isaia F, Lippolis V, Verani G (1994) Inorg Chem 33:6315–6324
Acknowledgements
We are very grateful to the NRF and the Centre of Excellence in Catalysis (c* change, PAR-08) for generous financial support. We thank Mr S.A. Ogundare for assistance with UV–Vis and fluorescence spectroscopic data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mncube, S.G., Zamisa, S.J. & Bala, M.D. Interhalogen 1,2,3-Triazolium Ionic Liquid: Synthesis, Crystal Structure, Hirshfeld Surface Analysis and Photophysical Properties. J Chem Crystallogr 52, 242–250 (2022). https://doi.org/10.1007/s10870-021-00912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00912-2