Abstract
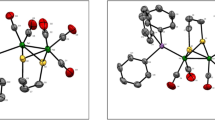
The 2:1 molar reaction of [Fe2(CO)6{(µ-SCH2)2CH2}] (1) and a bidentate diphosphine ligand, 1,6-bis(diphenylphosphino)hexane (dpph), in the presence of Me3NO resulted in the isolation of [Fe2(CO)5{Ph2P(CH2)3}{(µ-SCH2)2CH2}]2 (2) as red crystals in 71 % yield. The dpph ligand in compound 2, coordinates two Fe2S2 subunits as bridging mode and hence makes a linkage between two [Fe2(CO)5{(µ-SCH2)2CH2}] fragments. Each Fe2S2 butterfly of the complex consists of two fused Fe(S-C-C-C-S) six-membered rings, one of which attains a chair conformation, and the other is necessarily in a boat conformation. Compound 2 has unequivocally been characterized by elemental analysis, IR, 1H NMR and 31P NMR spectroscopy together with single crystal X-ray diffraction studies. Crystals of 2 are triclinic, space group P-1, a = 9.918(4) Å, b = 10.347(3) Å, c = 14.581(5) Å, α = 72.239(12)º, β = 80.342(12)º, γ = 68.550(14)º and Z = 1.
Graphic Abstract
A novel tetranuclear propane-1,3-dithiolate complex, [Fe2(CO)5{Ph2P(CH2)3}{(μ-SCH2)2CH2}]2 (2), bearing a bridging bis(diphenylphosphino)hexane (dpph) ligand, was synthesized from the Me3NO aided room temperature displacement of carbonyls from [Fe2(CO)6{(μ-SCH2)2CH2}] (1), and structurally characterized.




Similar content being viewed by others
Data Availability
Crystallographic data for the structural analysis of compound 2 have been deposited to the Cambridge Crystallographic Data Centre with CCDC number 2074713. These data may be obtained free of charge from The Director, Cambridge Crystallographic Database Centre, 12 Union Road Cambridge, CB2 1EZ, United Kingdom. (http://www.ccdc.cam.ac.uk).
References
Tard C, Pickett CJ (2009) Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem Rev 109:2245–2274. https://doi.org/10.1021/cr800542q
Gloaguen F, Rauchfuss TB (2009) Small molecule mimics of hydrogenases: Hydrides and redox. Chem Soc Rev 38:100–108. https://doi.org/10.1039/b801796b
Lubitz W, Ogata H, Rüdiger O, Reijerse E (2014) Hydrogenases Chem Rev 114:4081–4148
Rauchfuss TB (2015) Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere. Acc Chem Res 48:2107–2116. https://doi.org/10.1021/acs.accounts.5b00177
Jiang S, Zhang T, Zhang X et al (2015) Nitrogen heterocyclic carbene containing pentacoordinate iron dicarbonyl as a [Fe]-hydrogenase active site model. Dalton Trans 44:16708–16712. https://doi.org/10.1039/c5dt02065d
Peters JW (1998) X-ray Crystal Structure of the Fe-Only Hydrogenase (CpI) from Clostridium pasteurianum to 1.8 Angstrom Resolution. Science 282:1853–1858. https://doi.org/10.1126/science.282.5395.1853
Nicolet Y, Piras C, Legrand P et al (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7:13–23. https://doi.org/10.1016/S0969-2126(99)80005-7
Zheng D, Wang M, Chen L et al (2014) The influence of a S-to-S bridge in diiron dithiolate models on the oxidation reaction: A mimic of the Hairox state of [FeFe]-hydrogenases. Chem Commun 50:9255–9258. https://doi.org/10.1039/c4cc03583f
Ghosh S, Hogarth G, Hollingsworth N et al (2014) Hydrogenase biomimetics: Fe2(CO)4(µ-dppf)(µ-pdt) (dppf = 1,1′-bis(diphenylphosphino)ferrocene) both a proton-reduction and hydrogen oxidation catalyst. Chem Commun 50:945–947. https://doi.org/10.1039/c3cc46456c
Angamuthu R, Chen C-S, Cochrane TR et al (2015) N-substituted derivatives of the Azadithiolate cofactor from the [FeFe] hydrogenases: Stability and complexation. Inorg Chem 54:5717–5724. https://doi.org/10.1021/acs.inorgchem.5b00290
Gilbert-Wilson R, Siebel JF, Adamska-Venkatesh A et al (2015) Spectroscopic investigations of [FeFe] hydrogenase maturated with [ 57Fe2 (adt)(CN)2(CO)4]2–. J Am Chem Soc 137:8998–9005. https://doi.org/10.1021/jacs.5b03270
Wang W, Rauchfuss TB, Zhu L, Zampella G (2014) New reactions of terminal hydrides on a Diiron Dithiolate. J Am Chem Soc 136:5773–5782. https://doi.org/10.1021/ja501366j
Lyon EJ, Georgakaki IP, Reibenspies JH, Darensbourg MY (1999) Carbon monoxide and cyanide ligands in a classical organometallic complex model for Fe-only hydrogenase. Angew Chemie - Int Ed 38:3178–3180.
Schmidt M, Contakes SM, Rauchfuss TB (1999) First generation analogues of the binuclear site in the Fe-only hydrogenases: Fe2(µ-SR)2(CO)4(CN)22-. J Am Chem Soc 121:9736–9737. https://doi.org/10.1021/ja9924187
Li H, Rauchfuss TB (2002) Iron carbonyl sulfides, formaldehyde, and amines condense to give the proposed azadithiolate cofactor of the Fe-only hydrogenases. J Am Chem Soc 124:726–727. https://doi.org/10.1021/ja016964n
Ott S, Kritikos M, Åkermark B et al (2004) A biomimetic pathway for hydrogen evolution from a model of the iron hydrogenase active site. Angew Chemie Int Ed 43:1006–1009. https://doi.org/10.1002/anie.200353190
Seyferth D, Henderson RS, Song LC (1980) The dithiobis(tricarbonyliron) dianion: Improved preparation and new chemistry. J Organomet Chem 192:C1.
Seyferth D, Henderson RS, Song L (1982) Chemistry of dithio-bis(tricarbonyliron), a mimic of inorganic disulfides. Organometallics 1:125–133
Seyferth D, Womack GB, Gallagher MK et al (1987) Novel anionic rearrangements in hexacarbonyldiiron complexes of chelating organosulfur ligands. Organometallics 6:283–294. https://doi.org/10.1021/om00145a009
Zhao X, Georgakaki IP, Miller ML et al (2001) H/D exchange reactions in dinuclear iron thiolates as activity assay models of Fe–H2 ase. J Am Chem Soc 123:9710–9711. https://doi.org/10.1021/ja0167046
Li P, Wang M, He C et al (2005) Influence of tertiary phosphanes on the coordination configurations and electrochemical properties of iron hydrogenase model complexes: crystal structures of [(µ-S2C3H6)Fe2(CO)6–nLn] (L = PMe2Ph, n = 1, 2; PPh3, P(OEt)3, n = 1). Eur J Inorg Chem 2005:2506–2513. https://doi.org/10.1002/ejic.200400947
Zhao X, Georgakaki IP, Miller ML et al (2002) Catalysis of H2/D2 Scrambling and Other H/D Exchange Processes by [Fe]-Hydrogenase Model Complexes. Inorg Chem 41:3917–3928. https://doi.org/10.1021/ic020237r
Yan L, Li X, Yang J et al (2020) Diiron propane-1,3-dithiolate complexes with monosubstituted tri(m-tolyl)phosphine or tris(3-fluorophenyl)phosphine: synthesis, characterization, crystal structures, and electrochemistry. Mol Cryst Liq Cryst 702:54–63. https://doi.org/10.1080/15421406.2020.1743938
Li P, Wang M, Chen L et al (2009) Structures, protonation, and electrochemical properties of diiron dithiolate complexes containing pyridyl-phosphine ligands. J Chem Soc Dalton Trans 1919–1926. https://doi.org/10.1039/b814336f
Li P, Wang M, Chen L et al (2008) Supramolecular self-assembly of a [2Fe2S] complex with a hydrophilic phosphine ligand. CrystEngComm 10:267–269. https://doi.org/10.1039/b713159c
Gao S, Guo H, Peng X et al (2013) The employment of a hydrophilic tris(morpholino)phosphine ligand in diiron propane-1,3-dithiolate complexes for potentially water-soluble iron-only hydrogenase active-site mimics. New J Chem 37:1437–1444. https://doi.org/10.1039/c3nj41058g
Song L-C, Li C-G, Ge J-H et al (2008) Synthesis and structural characterization of the mono- and diphosphine-containing diiron propanedithiolate complexes related to [FeFe]-hydrogenases. Biomimetic H2 evolution catalyzed by (µ-PDT)Fe2(CO)4[(Ph2P)2N(n-Pr)]. J Inorg Biochem 102:1973–1979. https://doi.org/10.1016/j.jinorgbio.2008.04.003
Yan L, Wang L-H, Yang J et al (2020) Diiron propane-1,2-dithiolate complexes with monosubstituted tris(3-chlorophenyl)phosphine or tris(4-trifluoromethylphenyl)phosphine: synthesis, characterization, crystal structures, and electrochemistry. Inorg Nano-Metal Chem 50:1137–1143. https://doi.org/10.1080/24701556.2020.1735431
Islam S, Hossain MI, Karim MM, Bhoumik NC (2021) Carbonyl displacement reaction in Diiron propane-dithiolate complex by Triphenylstibine: crystal structure of [Fe2(CO)6-n(SbPh3)n(µ-S2C3H6)] (n = 1 and 2). J Chem Crystallogr. https://doi.org/10.1007/s10870-021-00884-3
De Beer JA, Haines RJ, Greatrex R, Greenwood NN (1971) Stereochemistry of the bis-substituted derivatives of bis-(µ-alkyl- and -phenylsulphidotricarbonyliron). J Organomet Chem 27:C33–C35.
Wang N, Wang M, Liu T et al (2008) CO-migration in the ligand substitution process of the celating diphosphite Diiron complex (µ-pdt)[Fe(CO)3][Fe(CO){(EtO)2PN(Me)P(OEt)2}]. Inorg Chem 47:6948–6955. https://doi.org/10.1021/ic800525n
Rana S, Ghosh S, Hossain MK et al (2016) Hydrogenase biomimetics: structural and spectroscopic studies on diphosphine-substituted derivatives of Fe2(CO)6(µ-edt) (edt = ethanedithiolate) and Fe2(CO)6(µ-tdt) (tdt = 1,3-toluenedithiolate). Transit Met Chem 41:933–942. https://doi.org/10.1007/s11243-016-0097-5
Liu Y-C, Tu L-K, Yen T-H et al (2011) Influences on the rotated structure of diiron dithiolate complexes: electronic asymmetry vs. secondary coordination sphere interaction. Dalton Trans 40:2528. https://doi.org/10.1039/c0dt01332c
Gao W, Ekstrǒm J, Liu J et al (2007) Binuclear iron-sulfur complexes with bidentate phosphine ligands as active site models of Fe-hydrogenase and their catalytic proton reduction. Inorg Chem 46:1981–1991. https://doi.org/10.1021/ic0610278
Justice AK, Zampella G, De Gioia L et al (2007) Chelate control of Diiron(I) dithiolates relevant to the [Fe–Fe]- hydrogenase active site. Inorg Chem 46:1655–1664. https://doi.org/10.1021/ic0618706
Adam FI, Hogarth G, Richards I (2007) Models of the iron-only hydrogenase: Reactions of [Fe2(CO)6(µ-pdt)] with small bite-angle diphosphines yielding bridge and chelate diphosphine complexes [Fe2(CO)4(diphosphine)(µ-pdt)]. J Organomet Chem 692:3957–3968. https://doi.org/10.1016/j.jorganchem.2007.05.050
Rohman MM et al (2020) A diiron propane-1,3-dithiolate complex [Fe2(CO)4(κ2-dpbp){µ-(SCH2)2CH2}] with a chelating dpbp [dpbp= 2,2´-bis(diphenyl phosphino)-1,1´-biphenyl] ligand. J Bangladesh Chem Soc 32:36–40
Adam FI, Hogarth G, Kabir SE, Richards I (2008) Models of the iron-only hydrogenase: Synthesis and protonation of bridge and chelate complexes [Fe2(CO)4{Ph2P(CH2)nPPh2}(µ-pdt)] (n = 2-4) - evidence for a terminal hydride intermediate. Comptes Rendus Chim 11:890–905. https://doi.org/10.1016/j.crci.2008.03.003
Liu X-F (2016) Synthesis and structures of diiron dithiolate complexes with 1,2-bis(diphenylphosphino)acetylene or tris(2-methoxyphenyl)phosphine. Polyhedron 117:672–678. https://doi.org/10.1016/j.poly.2016.07.009
Song LC, Wang HT, Ge JH et al (2008) Investigations on the active site models of [FeFe]-hydrogenases: synthesis, structure, and properties of N-functionalized azadithiolatodiiron complexes containing mono- And diphosphine ligands. Organometallics 27:1409–1416. https://doi.org/10.1021/om700956e
Ezzaher S, Capon J-F, Gloaguen F et al (2007) Evidence for the formation of terminal hydrides by protonation of an asymmetric iron hydrogenase active site mimic. Inorg Chem 46:3426–3428. https://doi.org/10.1021/ic0703124
Liu XF, Gao HQ (2014) Synthesis and crystal structures of Diiron dithiolate complexes containing Diphosphine ligands. J Clust Sci 25:495–503. https://doi.org/10.1007/s10876-013-0627-7
Liu XF, Yin BS (2010) Synthesis and structural characterization of a diiron propanedithiolate complex [(µ-PDT)Fe2(CO)5]2[(η5-Ph2PC5H4)2Fe] containing a bidentate phosphine ligand 1,1’-bis(diphenylphosphino)ferrocene. J Coord Chem 63:4061–4067. https://doi.org/10.1080/00958972.2010.531715
Li CG, Xue F, Cui MJ et al (2015) 1,1′-Bis(diphenylphosphino)ferrocene as an intramolecular or intermolecular bridging ligand related to the phenyl-functionalized diiron propanedithiolate complex: Synthesis and catalysis of the reduction of protons. Transit Met Chem 40:47–52. https://doi.org/10.1007/s11243-014-9888-8
Greco C (2013) H2 binding and splitting on a new-generation [FeFe]-hydrogenase model featuring a redox-active Decamethylferrocenyl phosphine ligand: a theoretical investigation. Inorg Chem 52:1901–1908. https://doi.org/10.1021/ic302118h
Camara JM, Rauchfuss TB (2012) Combining acid–base, redox and substrate binding functionalities to give a complete model for the [FeFe]-hydrogenase. Nat Chem 4:26–30. https://doi.org/10.1038/nchem.1180
Tard C, Liu X, Ibrahim SK et al (2005) Synthesis of the H-cluster framework of iron-only hydrogenase. Nature 433:610–613. https://doi.org/10.1038/nature03298
Li Y, Rauchfuss TB (2016) Synthesis of Diiron(I) dithiolato carbonyl complexes. Chem Rev 116:7043–7077. https://doi.org/10.1021/acs.chemrev.5b00669
Weigand W, Schollhammer P (2014) Bioinspired Catalysis. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Scott RA (2011) Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons, Inc., pp 1–21
Tschierlei S, Ott S, Lomoth R (2011) Spectroscopically characterized intermediates of catalytic H2 formation by [FeFe] hydrogenase models. Energy Environ Sci 4:2340. https://doi.org/10.1039/c0ee00708k
Felton GAN, Mebi CA, Petro BJ et al (2009) Review of electrochemical studies of complexes containing the Fe2S2 core characteristic of [FeFe]-hydrogenases including catalysis by these complexes of the reduction of acids to form dihydrogen. J Organomet Chem 694:2681–2699. https://doi.org/10.1016/j.jorganchem.2009.03.017
Gloaguen F (2016) Electrochemistry of simple organometallic models of iron–iron hydrogenases in organic solvent and water. Inorg Chem 55:390–398. https://doi.org/10.1021/acs.inorgchem.5b02245
Ezzaher S, Capon J-F, Gloaguen F et al (2009) Influence of a Pendant Amine in the Second Coordination Sphere on Proton Transfer at a Dissymmetrically Disubstituted Diiron System Related to the [2Fe]H Subsite of [FeFe]H2 ase. Inorg Chem 48:2–4. https://doi.org/10.1021/ic801369u
Wang N, Wang M, Liu J et al (2009) Preparation, Facile Deprotonation, and Rapid H/D Exchange of the µ-Hydride Diiron Model Complexes of the [FeFe]-Hydrogenase Containing a Pendant Amine in a Chelating Diphosphine Ligand. Inorg Chem 48:11551–11558. https://doi.org/10.1021/ic901154m
Ezzaher S, Capon J-F, Dumontet N et al (2009) Electrochemical study of the role of a H-bridged, unsymmetrically disubstituted diiron complex in proton reduction catalysis. J Electroanal Chem 626:161–170. https://doi.org/10.1016/j.jelechem.2008.12.005
Ghosh S, Sanchez BE, Richards I et al (2016) Biomimetics of the [FeFe]-hydrogenase enzyme: Identification of kinetically favoured apical-basal [Fe2(CO)4(µ-H){κ2-Ph2PC(Me2)PPh2}(µ-pdt)]+ as a proton-reduction catalyst. J Organomet Chem 812:247–258. https://doi.org/10.1016/j.jorganchem.2015.09.036
Ghosh S, Hogarth G, Hollingsworth N et al (2013) Models of the iron-only hydrogenase: a comparison of chelate and bridge isomers of Fe2(CO)4{Ph2PN(R)PPh2}(µ-pdt) as proton-reduction catalysts. Dalton Trans 42:6775. https://doi.org/10.1039/c3dt50147g
Adam FI, Hogarth G, Richards I, Sanchez BE (2007) Models of the iron-only hydrogenase: Structural studies of chelating diphosphine complexes [Fe2(CO)4(µ-pdt)(κ2-P,P′-diphosphine)]. Dalton Trans 2495–2498. https://doi.org/10.1039/B706123B
Ezzaher S, Capon J-F, Gloaguen F et al (2007) Electron-transfer-catalyzed rearrangement of unsymmetrically substituted Diiron Dithiolate complexes related to the active site of the [FeFe]-hydrogenases. Inorg Chem 46:9863–9872. https://doi.org/10.1021/ic701327w
Ridley F, Ghosh S, Hogarth G et al (2013) Fluorinated models of the iron-only hydrogenase: An electrochemical study of the influence of an electron-withdrawing bridge on the proton reduction overpotential and catalyst stability. J Electroanal Chem 703:14–22. https://doi.org/10.1016/j.jelechem.2013.05.018
Bruker (2015) SAINT (837A), Bruker AXS Inc, Madison, Wisconsin, USA
Bruker (2014) SADABS-2014/5, Bruker AXS Inc., Madison, Wisconsin, USA
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122. https://doi.org/10.1107/S0108767307043930
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
van der Sluis P, Spek AL (1990) BYPASS: an effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr A 46:194–201. https://doi.org/10.1107/S0108767389011189
Durgaprasad G, Bolligarla R, Das SK (2011) Synthesis, structural characterization and electrochemical studies of [Fe2(µ-L)(CO)6] and [Fe2(µ-L)(CO)5(PPh3)] (L = pyrazine-2,3-dithiolate, quinoxaline-2,3-dithiolate and pyrido[2,3-b]pyrazine-2,3-dithiolate): Towards modeling the active site of [FeFe]–. J Organomet Chem 696:3097–3105. https://doi.org/10.1016/j.jorganchem.2011.06.007
Aroyo MI (2016) International Tables for Crystallography. Vol. A, Chapter 2.3, P. 195, Chester, England
Acknowledgements
The authors acknowledge the University Grant Commission, Government of the People’s Republic of Bangladesh and Jahangirnagar University, for financial support, and Mr. Md. Emdad Hossain, Scientist, Wazed Miah Science Research Centre, Jahangirnagar University, for recording IR and 1H NMR spectra of the complex.
Author information
Authors and Affiliations
Contributions
The draft manuscript was prepared by the corresponding author (SI). All authors contributed to edit the manuscript except MMK since he passed away while the research work was in progress. The final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rohman, M.M., Hossain, M.I., Bhoumik, N.C. et al. A Tetranuclear Propane-1,3-Dithiolate Complex, [Fe2(CO)5{Ph2P(CH2)3}{(µ-SCH2)2CH2}]2, with a Bridging 1,6-Bis(diphenylphosphino)hexane Ligand. J Chem Crystallogr 52, 223–232 (2022). https://doi.org/10.1007/s10870-021-00910-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00910-4