Abstract
The title compound, 4-(4-methoxyphenethyl)-5-(p-tolyl)-2,4-dihydro-3H-1,2,4-triazol-3-one (C18H19N3O2), was characterized by single crystal X-ray diffraction. In order to compare the experimental and theoretical compatibility, the DFT and HF modeling technique was also used. When the theoretical and experimental results are compared, it is seen that the geometric parameters from both investigation techniques are quite compatible. X-ray diffraction (XRD) analysis shows that the structure has crystallized in the orthorhombic space group Pna21. The planes of the triazole and benzyl rings in the molecule make dihedral angles of 46.14(1)ο (C1-C6) and 43.89(1)ο (C12-C17). The molecules in the asymmetric unit are linked by intermolecular N–H···O hydrogen bonds, forming a three-dimensional network. Frontier molecular orbital (FMO) analysis was performed to describe intramolecular interactions. For the molecule the energy difference between the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) was calculated as − 4.95 eV. Potential energy surface (PES) analysis was performed by a semi-empirical method to determine the stable states of molecular structure and to compare them with XRD geometry. In order to determine the thermodynamic properties of the molecular structure enthalpy, heat capacity and entropy values were calculated for selected temperature values.
Graphical Abstract
Triazole compound were synthesized and characterized by elemental analyses, Molecular Hirshfeld Surface, Frontier Molecular Orbitals, Potential Energy Surface, Thermodynamic Properties analysis, and single crystal X-ray diffraction.

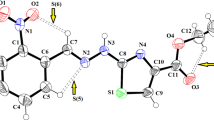
Figure drawing of the C18H19N3O2 molecule










Similar content being viewed by others
References
Malani AH, Makwana AH, Makwana HR (2017) A brief review article: Various synthesis and therapeutic importance of 1, 2, 4-triazole and its derivatives. Moroccan J Chem 5(1):5–1. https://doi.org/10.48317/IMIST.PRSM/morjchem-v5i1.5959
Kokil GR, Rewatkar PV, Gosain S, Aggarwal S, Verma A, Kalra A (2010) Synthesis and in vitro evaluation of novel 1, 2, 4-triazole derivatives as antifungal agents. Lett Drug Des Discovery 7(1):46–49. https://doi.org/10.2174/157018010789869415
Yengoyan AP, Pivazyan VA, Chazaryan EA, Hakobyan RS (2019) Synthesis of new thiazolo[3,2-b][1,2,4]triazole derivatives and preliminary evaluation of their biological activity. Russ J Gen Chem 89(1):32–36. https://doi.org/10.1134/S1070363219010067
Liu CJ, Liu YP, Yu SL, Dai XJ, Zhang T (2016) Syntheses, cytotoxic activity evaluation and HQSAR study of 1,2,3-triazole-linked isosteviol derivatives as potential anticancer agents. Bioorg Med Chem Lett 26(22):5455–5461. https://doi.org/10.1016/j.bmcl.2016.10.028
Kharb R, Sharma PC, Yar MS (2011) Pharmacological significance of triazole scaffold. J Enzyme Inhib Med Chem 26(1):1–21. https://doi.org/10.3109/14756360903524304
Ünver Y, Deniz S, Çelik F, Akar Z, Küçük M, Sancak K (2016) Synthesis of new 1, 2, 4-triazole compounds containing Schiff and Mannich bases (morpholine) with antioxidant and antimicrobial activities. J Enzyme Inhib Med Chem 31(sup3):89–95. https://doi.org/10.1080/14756366.2016.1206088
Ünver Y, Bektaş E (2018) Synthesis and biological activity of new Schiff bases of benzylideneamine bearing thiophene, 1, 2, 4-triazolone, 1, 3, 4-oxadiazole, morpholine moieties. Lett Drug Des Discovery 15(7):706–712. https://doi.org/10.2174/1570180814666171012163651
Ünver Y, Bektaş E, Direkel Ş (2018) Synthesis, antioxidant, and antileishmanial activities of new Bis-N-amino triazole derivatives. Russ J Gen Chem 88(12):2616–2620. https://doi.org/10.1134/S1070363218120241
Ünver Y, Tanak H (2018) Crystal structure of 4-Amino-3-(thiophen-2-ylmethyl)-1H-1, 2, 4-triazole-5 (4H) one monohydrate. Crystallogr Rep 63(4):585–588. https://doi.org/10.1134/S1063774518040302
Unver Y, Meydanal S, Sancak K, Unluer D, Ustabas R, Dugdu E (2011) Synthesis, crystal structure, and antioxidant properties of novel 1, 2, 4-triazol-5-ones containing 3, 4-dimethoxyphenyl and 3, 4-dihydroxyphenyl moiety. Turk J Chem 35(2):265–277. https://doi.org/10.3906/kim-1006-707
Keri RS, Patil SA, Budagumpi S, Nagaraja BM (2015) Triazole: a promising antitubercular agent. Chem Biol Drug Des 86(4):410–423. https://doi.org/10.1111/cbdd.12527
Kaur R (2016) Ashish Ranjan Dwivedi, Bhupinder Kumar, Vinod Kumar, Recent Developments on 1,2,4-Triazole Nucleus in Anticancer Compounds: A Review. Anticancer Agents Med Chem 16(4):465–489. https://doi.org/10.2174/1871520615666150819121106
Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S (2012) The biology and chemistry of antifungal agents: a review. Bioorg Med Chem 20(19):5678–5698. https://doi.org/10.1016/j.bmc.2012.04.045
Demirbaş Ü, Kobak RZU, Akçay HT, Ünlüer D, Koca A, Çelik F, Kantekin H (2016) Synthesis, characterization, electrochemical and spectroelectrochemical properties of novel peripherally tetra-1, 2, 4-triazole substituted phthalocyanines. Synth Met 215:68–76. https://doi.org/10.1016/j.synthmet.2016.02.004
Agilent (2011). CrysAlisPRO and CrysAlis RED. Agilent Technologies. Yarntone,England.
Sheldrick GM (2015) SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst A71:3–8
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Farrugia LJ (2012) J Appl Crystallogr 45:849. https://doi.org/10.1107/S0021889812029111
Becke AD (1993) J Chem Phys 98(7):5648. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1998) Phys Rev B 37(2):785. https://doi.org/10.1103/PhysRevB.37.785
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3(2):214–218. https://doi.org/10.1002/jcc.540030212
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J Comput Chem 17(1):49–56. https://doi.org/10.1002/(SICI)1096-987X(19960115)17:1%3c49::AID-JCC5%3e3.0.CO;2-0
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03. Wallingford Conn, USA
Dennington R, Keith T, Millam J (2007) GaussView, Version 4.1. Semichem Inc, Shawnee Mission Kan USA
M.J. Turner, J.J. MacKinnon, S.K. Wolff, D.J. Grimwood, P.R. Spackman, D. Jayatilaka, and M.A. Spackman, Crystal Explorer17.5. University of Western Australia. (2017). http://hirshfeldsurface.net.
Hanif M, Qadeer G, Rama NH, Akhtar J, Helliwell M (2009) 4-(3-Methoxyphenyl)-3-[2-(4-methoxyphenyl) ethyl]-1H-1, 2, 4-triazol-5 (4H)-one. Acta Crystallogr Sect E: Struct Rep Online 65(2):o387–o387. https://doi.org/10.1107/S1600536809002840
Köysal Y, Tanak H (2012) Crystal structure, spectroscopic investigations and density functional studies of 4-(4-methoxyphenethyl)-5-benzyl-2H-1, 2, 4-triazol-3 (4H)-one monohydrate. Spectrochim Acta Part A Mol Biomol Spectrosc 93:106–115. https://doi.org/10.1016/j.saa.2012.02.054
Öztürk Yildirim S, Butcher RJ, Ünlüer D, Köysal Y (2012) 4-[2-(4-Hydroxyphenyl) ethyl]-3-propyl-1H-1, 2, 4-triazol-5 (4H)-one. Acta Crystallogr Sect E: Struct Rep Online 68(6):1651–1652. https://doi.org/10.1107/S1600536812019447
Köysal Y, Tanak H, Ünlüer D, Işık Ş (2010) 4-(4-Methoxyphenethyl)-3-methyl-1H-1, 2, 4-triazol-5 (4H)-one. Acta Crystallogr Sect E: Struct Rep Online 66(8):o2158–o2158. https://doi.org/10.1107/S1600536810029685
Gurumoorthy A, Gopalsamy V, Ramamurthi K, Ünlüer D, Celik F (2011) 4-[2-(4-Methoxyphenyl) ethyl]-3-(thiophen-2-ylmethyl)-1H-1, 2, 4-triazol-5 (4H)-one monohydrate. Acta Crystallogr Sect E: Struct Rep Online 67(12):o3188–o3189. https://doi.org/10.1107/S1600536811045508
Bülbül H, Yıldırım SÖ, Köysal Y, Ünlüer D, Soylu MS, Butcher RJ (2020) Crystal Structure and Computational Study of 5-Ethyl-4-(4-Methoxyphenethyl)-4, 5-Dihydro-3 H-1, 2, 4-Triazol-3-One and 4-(4-Methoxyphenethyl)-5-Propyl-4, 5-Dihydro-3 H-1, 2, 4-Triazol-3-One. Crystallogr Rep 65(7):1133–1137. https://doi.org/10.1134/S1063774520070044
Aydemir E, Kansız S, Gümüş MK, Gorobets NY, Dege N (2018) Crystal structure and Hirshfeld surface analysis of 7-ethoxy-5-methyl-2-(pyridin-3-yl)-11, 12-dihydro-5, 11-methano [1, 2, 4] triazolo [1, 5-c][1, 3, 5] benzoxadiazocine. Acta Crystallographica Section E: Crystallographic Communications 74(3):367–370. https://doi.org/10.1107/s2056989018002621
Gümüş MK, Kansız S, Aydemir E, Gorobets NY, Dege N (2018) Structural features of 7-methoxy-5-methyl-2-(pyridin-3-yl)-11, 12-dihydro-5, 11-methano [1, 2, 4] triazolo [1, 5-c][1, 3, 5] benzoxadiazocine: Experimental and theoretical (HF and DFT) studies, surface properties (MEP, Hirshfeld). J Mol Struct 1168:280–290. https://doi.org/10.1016/j.molstruc.2018.05.032
Hökelek T, Özkaya S, Necefoğlu H (2018) Crystal structure and Hirshfeld surface analysis of diaquabis (N, N-diethylnicotinamide-κN1) bis (2, 4, 6-trimethylbenzoato-κO) manganese (II). Acta Crystallographica Section E: Crystallographic Communications 74(4):422–427. https://doi.org/10.1107/S2056989018003377
Kansız S, Dege N (2018) Synthesis, crystallographic structure, DFT calculations and Hirshfeld surface analysis of a fumarate bridged Co (II) coordination polymer. J Mol Struct 1173:42–51. https://doi.org/10.1016/j.molstruc.2018.06.071
Fukui K (1982) Role of frontier orbitals in chemical reactions. Science 218(4574):747–754
Tanak H, Pawlus K, Marchewka MK, Pietraszko A (2014) Structural, vibrational and theoretical studies of anilinium trichloroacetate: New hydrogen bonded molecular crystal with nonlinear optical properties. Spectrochim Acta Part A Mol Biomol Spectrosc 118:82–93. https://doi.org/10.1016/j.saa.2013.08.027
Acknowledgements
RJB acknowledges the NSF–MRI program (Grant No. CHE-0619278) for funds to purchase the diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bülbül, H., Köysal, Y., Yıldırım, S.Ö. et al. Crystal Structure, Computational Study and Hirshfeld Surface Analysis of 4-(4-Methoxyphenethyl)-5-(p-tolyl)-2,4-Dihydro-3H-1,2,4-Triazol-3-One, C18H19N3O2. J Chem Crystallogr 52, 440–449 (2022). https://doi.org/10.1007/s10870-021-00909-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00909-x