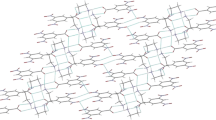
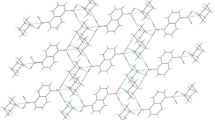
Abstract
Five crystalline organic acid-base salts [(HL)·(dnsa−), L = 2,6-dimethylaniline, dnsa− = 3,5-dinitrosalicylate] (1), [(HL+)·(4-Hnpta−), 4-Hnpta− = 4-nitrophthalate] (2), [(HL)2·(nds)·(H2O)2, nds = 1,5-naphthalenedisulfonate] (3), [(HL)·(dnb)·(Hdnb), dnb = 3,5-dinitrobenzoate, Hdnb = 3,5-dinitrobenzoic acid] (4) and [(HL)·(dca), dca = dichloroacetate] (5) from 2,6-dimethylaniline and organic acids were prepared and characterized by XRD analysis, IR, mp and elemental analysis. Compound 1 adopts the triclinic, space group Pī, with a = 7.6261(6) Å, b = 8.3429(8) Å, c = 13.1147(12) Å, α = 91.3610(10)º, β = 102.755(2)º, γ = 102.597(2)º, V = 791.96(12) Å3, Z = 2. Compound 2 belongs to the monoclinic, space group P2(1)/c, with a = 14.2552(15) Å, b = 8.1436(8) Å, c = 14.5708(13) Å, α = 90°, β = 113.795(2)º, γ = 90°, V = 1547.7(3) Å3, Z = 4. Compound 3 crystallizes in the monoclinic, space group P2(1)/c, with a = 8.4784(7) Å, b = 17.4798(15) Å, c = 9.1119(8) Å, α = 90°, β = 99.742(2)º, γ = 90°, V = 1330.9(2) Å3, Z = 2. Compound 4 has orthorhombic, space group Pna2(1), with a = 24.5029(19) Å, b = 7.5322(9) Å, c = 26.665(2) Å, α = 90°, β = 90°, γ = 90°, V = 4921.3(8) Å3, Z = 8. Compound 5 crystallizes in the monoclinic, space group C2/c, with a = 19.8970(17) Å, b = 11.1850(11) Å, c = 13.1590(12) Å, α = 90°, β = 123.408(3)º, γ = 90°, V = 2444.6(4) Å3, Z = 8. For 1 it was the relatively weak phenol that has ionized, different from 2 to 5. All supramolecular architectures of 1–5 involve N–H⋯O H-bonds as well as CH3⋯O interactions. The other noncovalent interactions (CH⋯O, CH⋯Cl, O⋯C, O⋯N, O⋯O, Cl⋯Cl, C⋯π, O⋯π, CH3⋯π and π⋯π) in the crystal packing were also ascertained. These weak interactions combined, all compounds displayed 3D framework structures.
Graphical Abstract
In the five prepared supramolecular assemblies there are plenty of weak bonding interactions such as directional H-bonds of N–H⋯O, O–H⋯O, O–H⋯S, O–H⋯N and noncovalent bonds of CH⋯O, CH3⋯O, CH⋯Cl, O⋯C, O⋯N, O⋯O, Cl–Cl, C⋯π, O⋯π, CH3⋯π, and aryl⋯aryl interactions. All compounds displayed the 3D framework structures













Similar content being viewed by others
References
Metrangolo P, Neukirch H, Pilati T, Resnatti G (2005) ACC Chem Res 38:386
Britz DA, Khlobystov AN (2006) Chem Soc Rev 35:637
Moulton B, Zaworotko MJ (2001) Chem Rev 101:1629
Steiner T (2002) Angew Chem Int Ed 41:48
Kinbara K, Hashimoto Y, Sukegawa M, Nohira H, Saigo K (1996) J Am Chem Soc 118:3441
Soldatov DV, Moudrakovski IL, Grachev EV, Ripmeester JA (2006) J Am Chem Soc 128:6737
Seaton CC, Parkin A, Wilson CC, Bladen N (2009) Cryst Growth Des 9:47
Bazuin CG, Brandys FA (1992) Chem Mater 4:970
Desiraju GR, Steiner T (2001) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry. Marcel Dekker, New York
Steed JW, Atwood JL (2009) Supramolecular Chemistry, 2nd edn. Wiley, Chichester
MacDonald JC, Whitesides GM (1994) Chem Rev 94:2383
Mu Z, Shu L, Fuchs H, Mayor M, Chi L (2008) J Am Chem Soc 130:10840
Desiraju GR (2011) Angew Chem Int Ed Engl 50:52
Wang ZQ, Wang LY, Zhang X, Shen JC, Denzinger S, Ringsdorf H (1997) Macromol Chem Phys 198:573
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dhannenberg JJ, Hobza P, Kjaergaard HG, Legon AC, Mennucci B, Nesbitt DJ (2011) Pure Appl Chem 83:1619
Görbitz CH, Nilsen M, Szeto K, Tangen LW (2005) Chem Commun 34:4288
Du M, Zhang ZH, Guo W, Fu XJ (2009) Cryst Growth Des 9:1655
Kodama K, Kobayashi Y, Saigo K (2007) Cryst Growth Des 7:935
Braga D, Brammer L, Champness NR (2005) CrystEngComm 7:1
Biradha K (2003) CrystEngComm 5:374
Yao J, Chen JM, Xu YB, Lu TB (2014) Cryst Growth Des 14:5019
Chen JM, Li S, Lu TB (2014) Cryst Growth Des 14:6399
Wang ZZ, Chen JM, Lu TB (2012) Cryst Growth Des 12:4562
Gould PJ (1986) Int J Pharm 33:201
Geng N, Chen JM, Li ZJ, Jiang L, Lu TB (2013) Cryst Growth Des 13:3546
Sanphui P, Devi VK, Clara D, Malviya N, Ganguly S, Desiraju GR (2015) Mol Pharm 12:1615
Gu JK, Hill CL, Hu CW (2015) Cryst Growth Des 15:3707
Schultheiss N, Lorimer K, Wolfe S, Desper J (2010) CrystEngComm 12:742
Chow K, Tong HHY, Lum S, Chow AHL (2008) J Pharm Sci 97:2855
Childs SL, Zaworotko MJ (2009) Cryst Growth Des 9:4208
Rager T, Hilfiker R (2010) Cryst Growth Des 10:3237
Zheng SL, Chen JM, Zhang WX, Lu TB (2011) Cryst Growth Des 11:466
Tothadi S, Sanphui P, Desiraju GR (2014) Cryst Growth Des 14:5293
Patra R, Titi HM, Goldberg I (2013) Cryst Growth Des 13:1342
Aakeröy CB, Schultheiss NC, Rajbanshi A, Desper J, Moore C (2009) Cryst Growth Des 9:432
Gavezzotti A, Presti LL (2015) Cryst Growth Des 15:3792
Das D, Jetti RKR, Boese R, Desiraju GR (2003) Cryst Growth Des 3:675
Beyer T, Price SL (2000) J Phys Chem B 104:2647
Kuduva SS, Craig DC, Nangia A, Desiraju GR (1999) J Am Chem Soc 121:1936
Kolotuchin SV, Fenlon EE, Wilson SR, Loweth CJ, Zimmerman SC (1995) Angew Chem Int Ed Engl 34:2654
Sanphui P, Bolla G, Das U, Mukherjee AK, Nangia A (2013) CrystEngComm 15:34
Hursthouse MB, Montis R, Tizzard GJ (2011) CrystEngComm 13:3390
Das D, Desiraju GR (2006) CrystEngComm 8:674
Akiri K, Cherukuvada S, Rana S, Nangia A (2012) Cryst Growth Des 12:4567
Thanigaimani K, Khalib NC, Temel E, Arshad S, Razak IA (2015) J Mol Struct 1099:246
Men YB, Sun JL, Huang ZT, Zheng QY (2009) CrystEngComm 11:978
Highfill ML, Chandrasekaran A, Lynch DE, Hamilton DG (2002) Cryst Growth Des 2:15
Lou BY, Perumalla SR, Sun CQC (2015) J Mol Struct 1099:516
Nichol GS, Clegg W (2009) Cryst Growth Des 9:1844
Haynes DA, Pietersen LK (2008) CrystEngComm 10:518
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556
MacDonald JC, Dorrestein PC, Pilley MM (2001) Cryst Growth Des 1:29
Padmavathy R, Karthikeyan N, Sathya D, Jagan R, Kumar RM, Sivakumar K (2016) RSC Adv 6:68468
Wu DH, Ge JZ, Cai HL, Zhang W, Xiong RG (2011) CrystEngComm 13:319
Jones CL, Wilson CC, Thomas LH (2014) CrystEngComm 16:5849
Sivakumar PK, Kumar MK, Kumar RM, Chakkaravarthi G, Kanagadurai R (2015) Acta Cryst E71:o163
Smirani W, Amri O, Rzaigui M (2008) Acta Cryst E64:o2463
Tang JJ, Chen J, Wang JT, Lu AH, Chen YS (2008) Acta Cryst E64:o244
Jin SW, Zhang WB, Wang DQ, Gao HF, Zhou JZ, Chen RP, Xu XL (2010) J Chem Crystallogr 40:87
Jin SW, Wang DQ, Jin ZJ, Wang LQ (2009) Polish J Chem 83:1937
Jin SW, Zhang WB, Liu L, Gao HF, Wang DQ, Chen RP, Xu XL (2010) J Mol Struct 975:128
Jin SW, Zhang WB, Liu L, Wang DQ, He HD, Shi T, Lin F (2011) J Mol Struct 991:1
Jin SW, Liu L, Wang DQ, Guo JZ (2011) J Mol Struct 1005:59
Jin SW, Wang DQ, Wang XL, Guo M, Zhao QJ (2008) J Inorg Organomet Polym 18:300
Bruker (2004) SMART and SAINT. Bruker AXS, Madison
Sheldrick GM (2000) SHELXTL, Structure Determination Software Suite, version 6.14. Bruker AXS, Madison
Lynch DE, Thomas LC, Smith G, Byriel KA, Kennard CHL (1998) Aust J Chem 51:867
Smith G, White JM (2001) Aust J Chem 54:97
Jin SW, Guo M, Wang DQ (2012) J Mol Struct 1022:220
Smith G, Wermuth UD, Healy PC (2005) Acta Cryst E61:o746
Smith G, Wermuth UD, Healy PC, White JM (2011) J Chem Crystallogr 41:1649
Smith G, Wermuth UD (2014) Acta Cryst E70:430
Smith G, Wermuth UD, White JM (2001) Acta Cryst E57:o1036
Abid S, Hemissi H, Rzaigui M (2007) Acta Cryst E63:o3117
Wei SS, Jin SW, Hu ZF, Zhou Y, Zhou YP (2012) Acta Cryst E68:o3117
Smith G, Wermuth UD, White JM (2003) Acta Cryst E59:o1977
Jin SW, Zhang H, Zhao Y, Jin L, Ye XH, Liu H, Wang DQ (2015) J Mol Struct 1099:304
Glidewell C, Low JN, Skakle JMS, Wardell JL (2005) Acta Cryst C61:o276
Dale SH, Elsegood MRJ, Hemmings M, Wilkinson AL (2004) CrystEngComm 6:207
Wang YF (2012) Acta Cryst E68:o1619
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555
Sun W, Shan GZ (2015) Acta Cryst E71:o361
Acknowledgements
This research was supported by the Open Fund of Zhejiang Provincial Top Key Discipline of Forestry Engineering under Grant No. 2014LYGCZ017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, Z., Lu, Y., Jin, S. et al. Crystal and Molecular Structures of Five 3D Organic Salts from 2,6-Dimethylaniline and Organic Acids. J Chem Crystallogr 49, 245–259 (2019). https://doi.org/10.1007/s10870-018-0760-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0760-0