Abstract
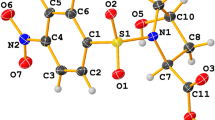
A series of co-crystals containing 5-fluorouracil as the active pharmaceutical ingredient were prepared via mechanochemical grinding and a normal solution method. Results indicate that both methods produced similar products, as verified by comparison of the X-ray powder diffraction patterns. Structural studies on this series of co-crystals revealed the non-ionic interactions present in the crystal lattice that form 1, 2, and 3-dimensional networks through persistent hydrogen bonds formed by certain functional groups; these may be used as templates to create new solid-state structures. Docking studies using the CDOCKER protocol in Discovery Studio Version 2.5 were used to investigate the potential anti-cancer activities of the novel co-crystals against a colorectal cancer target protein, human thymidylate synthase. The results were compared with a control ligand, dUMP, which is also found in the structure of the deposited protein model, 1HVY. A CDOCKER interaction energy of -34.65 kcal/mol compared to that of dUMP was calculated, indicating that these co-crystals are promising anti-cancer compounds.
Graphical Abstract
Developing a series of 5-fluorouracil co-crystals; synthesis, characterization and their potential anti-cancer from molecular docking. The colorectal cancer target protein used in this study, human thymidylate synthase (PDB: 1HVY, shown as a cartoon), showing the secondary structure. The ligands dUMP (red), co-crystals 1 (blue), 2 (green), 3 (yellow), and 4 (purple) are shown superimposed in the binding pocket.








Similar content being viewed by others
References
Delori A, Friščić T, Jones W (2012) CrystEngComm 14:2350–2362
Dunitz JD (1991) Pure Appl Chem 63:177–185
Zaworotko MJ (2007) Cryst Growth Des 7:4–9
Desiraju GR (2013) J Am Chem Soc 135:9952–9967
Thomas R, Kulkarni GU (2007) Beilstein J Org Chem 4:4–7
Desiraju GR (1995) Angew Chem Int Ed Engl 34:2311–2327
Etter MC (1990) Acc Chem Res 23:120–126
Braga D (2003) Chem Commun (Camb) 22:2751–2754
Aakeröy CB, Champness NR, Janiak C (2010) CrystEngComm 12:22–43
Nanjwade VK, Manvi FV, Shamrez AM, Nanjwade BK, Maste MM (2011) J Appl Pharm Sci 1:1–5
Bond AD (2007) CrystEngComm 9:833
Sekhon BS (2009) Ars Pharmaceutica 50:99–117
Aakeröy CB, Grommet AB, Desper J (2011) Pharmaceutics 3:601–614
Almarsson O, Zaworotko MJ (2004) Chem Commun (Camb) 17:1889–1896
Shan N, Zaworotko MJ (2008) Drug Discov Today 13:440–446
Blagden N, Berry DJ, Parkin A, Javed H, Ibrahim A, Gavan PT, De Matos LL, Seaton CC (2008) New J Chem 32:1659–1672
Raghuram M, Alam MS, Prasad M (2014) Khanduri CHAS 6:1–5
Sharma V, Chitranshi N, Agarwal AK (2014) Int J Med Chem. doi:10.1155/2014/202784
Delori A, Eddleston MD, Jones W (2013) CrystEngComm 15:73–77
Sousa MML, Krokan HE (2007) Slupphaug G 28:276–306
Kavli B, Otterlei M, Slupphaug G (2006) Krokan HE 6:505–516
Xia B, Liu Y, Li W, Brice AR, Dominy BN, Cao W (2014) J Biol Chem 289:18413–18426
Krokan HE, Drabløs F, Slupphaug G (2002) Oncogene 21:8935–8948
Brockman RW, Davis JM, Stutts P (1960) Biochim Biophys Acta 40:22–32
Grem J (2000) Invest New Drugs 18:299–313
Zhang N, Yin Y, Xu SJ, Chen WS (2008) Molecules 13:1551–1569
Longley DB, Harkin DP, Johnston PG (2003) Nat Rev Cancer 3:330–338
Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J (1957) Nature 179:663–666
Wishkerman S, Bernstein J, Hickey MB (2009) Cryst Growth Des 9:3204–3210
Li S, Chen J-M, Lu T-B (2014) CrystEngComm 16:6450–6458
Barnett SA, Hulme AT, Tocher DA (2006) Acta Crystallogr. Sect C 2:412–415
Singh UP, Kashyap S, Singh HJ, Mishra BK, Roy P, Chakraborty A (2012) J Mol Struct 1014:47–56
Yang J, Li S, Zhao H, Song B, Zhang G, Zhang J, Zhu Y, Han J (2014) J Phys Chem A 118:10927–10933
Sheldrick GM (2007) Acta Crystallogr. Sect A 64:112–122
Dolomanov OV, Bourhis LJ, Gildea RJ, Puschmann H (2009) J Appl Crystallogr 42:339–341
Spek A (2009) Acta Crystallogr. Sect D 65:148–155
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Crystallogr 41:466–470
B. V PANalytical, X’Pert HighScore Plus, Version, 2009
Phan J, Koli S, Minor W, Dunlap RB, Berger SH, Lebioda L (2001) Biochemistry 40:1897–1902
Dassault Systèmes BIOVIA (2015) Discovery Studio Modeling Environment, Release 4.5, San Diego, Dassault Systèmes
Momany FA, Rone R (1992) J Comput Chem 13:888–900
Dai Y, Wang Q, Zhang X, Jia S, Zheng H, Feng D, Yu P (2010) Eur J Med Chem 45:5612–5620
Akalin E, Akyuz S, Akyuz T (2007) J Mol Struct 834:477–481
Alcolea Palafox M, Tardajos G, Guerrero-Martínez A, Vats JK, Joe H, Rastogi VK (2010) Spectrochim. Acta Part A 75:1261–1269
Bhogala BR, Captain B, Parthasarathy A, Ramamurthy V (2010) J Am Chem Soc 132:13434–13442
Janiak C (2000) J Chem Soc Dalton Trans 21:3885–3896
Winter G, Fersht AR, Wilkinson AJ, Zoller M, Smith M (1982) Nature 299:756–758
Wilkinson AJ, Fersht AR, Blow DM, Winter G (1983) Biochemistry 22:3581–3586
Wilkinson AJ, Fersht AR, Blow DM, Carter P, Winter G (1984) Nature 307:187–188
Acknowledgements
We appreciatively acknowledge Ministry of Higher Education of Malaysia and University of Malaya for the Exploratory Research Grant Scheme, ERGS (ER008-2013A), Fundamental Research Grant Scheme, FRGS (FP005-2015A), and Postgraduate Research Grant, PPP (PG054-2013B) and Computation and Informatics (C + i) Research Cluster/High Performance Scientific Computing Program (UMRG Project No. RP001C-13ICT) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nadzri, N.I., Sabri, N.H., Lee, V.S. et al. 5-Fluorouracil Co-crystals and Their Potential Anti-cancer Activities Calculated by Molecular Docking Studies. J Chem Crystallogr 46, 144–154 (2016). https://doi.org/10.1007/s10870-016-0638-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-016-0638-y