Abstract
Three new Schiff base derivatives of (2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)-phenylmethanone, namely, C23H21NO3S (I), C24H22N2O5S (II) and C26H28N2O2S (III) have been synthesized and characterized by NMR, single-crystal X-ray diffraction and DFT molecular orbital calculations. Compound (I) crystallizes in the orthorhombic space group P212121, with Z = 4 in cells with a = 5.02536(8) Å, b = 17.5927(3) Å, c = 21.2134(4) Å, V = 1875.47(5) Å3 and displays weak C–H···O intermolecular interactions which contribute to crystal packing. Compound (II) crystallizes in the monoclinic space group P21/c, with Z = 4 in cells with a = 11.2263(2) Å, b = 19.7401(3) Å, c = 10.0202(2) Å, β = 108.565(2)°, V = 2105.00(8) Å3 and displays weak C–H···O intermolecular interactions forming zig-zag chains along the b axis and weak π–π stacking interactions which influence crystal packing. Compound (III) also crystallizes in the monoclinic space group P21/c, with Z = 4 in cells with a = 10.9599(2) Å, b = 11.9287(3) Å, c = 17.0626(4) Å, β = 97.680(2)°, V = 2210.71(8) Å3 and displays weak C–H···O intermolecular interactions which contribute to crystal packing. Additionally, the DFT frontier molecular orbitals of each compound are displayed and correlation between the calculated molecular orbital energies (eV) for the surfaces of the frontier molecular orbitals to the electronic excitation transitions from the absorption spectra of each compound has been proposed.
Graphical Abstract
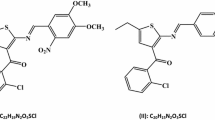
Synthesis, crystal structures, density functional theory (DFT) and molecular orbital surface calculations of three new Schiff base derivatives of (2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)-phenylmethanone: {2-[(2-hydroxy-3-methoxy-benzylidene)-amino]-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl}-phenylmethanone (I): C23H21NO3S; {2-[(4,5-dimethoxy-2-nitro-benzylidene)-amino]-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl}-phenylmethanone, (II): C24H22N2O5S and {2-[(4-diethylamino-2-hydroxybenzylidene)-amino]-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl}-phenylmethanone, (III): C26H28N2O2S.











Similar content being viewed by others
References
Molvi KI, Vasu KK, Yerande SG, Sudarsanam V, Haque N (2007) Eur J Med Chem 42:1049–1058
Rai NS, Kalluraya B, Lingappa B, Shenoy S, Puranic VG (2008) Eur J Med Chem 43:1715–1720
Asthalatha BV, Narayana B, Vijaya Raj KK, Kumari NS (2007) Eur J Med Chem 42:719–728
Sabnis RW, Rangnekar DW, Sonawane ND (1999) J Heterocycl Chem 36:333–345
Puterová Z, Krutošiková A, Végh D (2010) Arkivoc 1:209–246
Cannito A, Perrisin M, Luu-Duc C, Huguer F, Gaultier C, Narcisse G (1990) Eur J Med Chem 25:635–639
Nikolakopoulos G, Figler H, Linden J, Scammells P (2006) Bioorg Med Chem 14:2358–2365
Lütjens H, Zickgraf A, Figler H, Linden J, Olsson RA, Scammells PJ (2005) J Med Chem 46:1870–1877
Desai SB, Desai PB, Desai KR (2001) Hetrocycl Commun 7:83–90
Karia FD, Parsania PH (1999) Asian J Chem 11:991–995
Samadhiya S, Halve A (2001) Orient J Chem 17:119–122
Singh WM, Dash BC (1988) Pesticides 22:33–37
Aydogan F, Ocal N, Turgut Z, Yolacan C (2001) Bull Korean Chem Soc 22:476–480
Taggi AE, Hafez AM, Wack H, Young B, Ferraris D, Lectka T (2002) J Am Chem Soc 124:6626–6635
Kubicki M, Dutkiewicz G, Yathirajan HS, Dawar P, Ramesha AR, Dayananda AS (2012) Crystals 2:1058–1066
Kaur M, Jasinski JP, Kavitha CN, Yathirajan HS, Byrappa K (2014) Acta Cryst E70:o738–o739
Kaur M, Jasinski JP, Yamuna TS, Yathirajan HS, Byrappa K (2014) Acta Cryst E70:o501–o502
Kaur M, Jasinski JP, Kavitha CN, Yathirajan HS, Byrappa K (2014) Acta Cryst E70:o476–o477
Kaur M, Jasinski JP, Kavitha CN, Yathirajan HS, Byrappa K (2014) Acta Cryst E70:o507–o508
Kaur M, Jasinski JP, Yamuna TS, Yathirajan HS, Byrappa K (2014) Acta Cryst E70:o581–o582
Oxford Diffraction, CrysAlisPRO (2007) Version 171.31.8 and CrysAlisRED (2007) Version 1.171.31.8. Oxford Diffraction Ltd., Abingdon
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Palatinus L, Chapuis G (2007) J Appl Cryst 40:786–790
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Spek AL (2001) PLATON—a multipurpose crystallographic tool. Ultrecht University, Ultrecht
Johnson CK (1976) ORTEP II. Report ORNL-5138. Oak Ridge National Laboratory, Oak Ridge
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor RJ (1987) Chem Soc Perkin Trans 2:S1–S19
Schmidt JR, Polik WF (2007) WebMO Pro, version 8.0.01e; WebMO, LLc, Holland. http://www.webmo.net
Frisch MJ et al (2004) Gaussian 03, Revision C01. Gaussian Inc., Wallingford
Becke AD (1998) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Hehre WJ, Random L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Georgakopoulous S, Grondelle RV, Zwan GVD (2004) J Biophys 87:3010–3022
Guzin A (2002) Turk J Chem 26:295–302
IGOR Pro (1988–2009) WaveMetrics. Lake Oswego
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Acknowledgments
MK is grateful to CPEPA–UGC for the award of a JRF and thanks the University of Mysore for research facilities. JPJ acknowledges the NSF–MRI program (Grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, M., Jasinski, J.P., Anderson, B.J. et al. Synthesis, Crystal Structures and DFT Calculations of Three Schiff Base Derivatives of (2-Amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)-phenylmethanone. J Chem Crystallogr 46, 44–56 (2016). https://doi.org/10.1007/s10870-015-0626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0626-7