Abstract
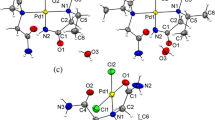
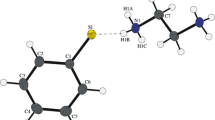
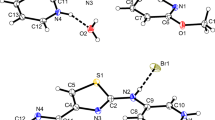
The crystal structures of the 1:1 proton-transfer compounds of the dipolar donor-π-acceptor compound N,N-dimethyl-4-[(E)-2-(4-pyridinyl)vinyl]aniline (1), also named dimethylamino-stilbazole, with 3-nitrophthalic and perchloric acids, viz. (E)-4-[4-(dimethylamino)styryl]pyridinium 2-carboxy-3-nitrobenzoate monohydrate (2) and (E)-4-[4-(dimethylamino)styryl]pyridinium perchlorate (3) are described. A redetermination of the structure of the parent free base 1 at 100 K is also reported. The (E)-4-[4-(dimethylamino)styryl]pyridinium ion exhibits slight whole molecule disorder in 2 and 3. Compound 2 is the first organic salt that contains the unusual monoanion of 3-nitrophthalic acid that is deprotonated at the 1-carboxyl group.
Graphical Abstract
The crystal structures of the 1:1 proton-transfer compounds of the dipolar donor-π-acceptor compound N,N-dimethyl-4-[(E)-2-(4-pyridinyl)vinyl]aniline with 3-nitrophthalic and perchloric acids, viz. (E)-4-[4-(dimethylamino)styryl]pyridinium 2-carboxy-3-nitrobenzoate monohydrate and (E)-4-[4-(dimethylamino)styryl]pyridinium perchlorate are described.








Similar content being viewed by others
References
Papadopoulos MG, Leszcynski J, Sadlej AJ (2006) Nonlinear optical properties of matter: from molecules to condensed phases. Springer, Dordrecht
Marder SR, Perry JW, Schaefer WP (1989) Science 245:626–628
Marder SR, Perry JW, Yakymyshyn CP (1994) Chem Mater 6:1137–1147
Coe BJ, Harris J, Asselberghs I, Wostyn K, Clays K, Persoons A, Brunschwig B, Coles SJ, Gelbrich T, Light ME, Hursthouse MB, Nakatani K (2003) Adv Funct Mater 13:347–357
Sliwa M, Létard S, Malfant I, Nierlich M, Lacroix PG, Asahi T, Masuhara H, Yu P, Nakatani K (2005) Chem Mater 17:4727–4735
Ruiz B, Coe BJ, Gianotti R, Gramlich V, Jazbinsek M, Günter P (2007) CrystEngComm 9:772–776
Yang Z, Wörle M, Mutter L, Jazbinsek M, Günter P (2007) Cryst Growth Des 7:83–86
Coe BJ, Foxon SP, Harper EC, Harris JA, Helliwell M, Raftery J, Asselberghs I, Clays K, Franz E, Brunschwig BS, Fitch AG (2009) Dyes Pigm 82:171–186
Asnis LN, Burunkova YE, Veniaminov AV, Knysh AS, Minozhenko OA (2011) J Opt Technol 78:761–764
Yin J, Li L, Yang Z, Jazbinsek M, Tao X, Günter P, Yang H (2012) Dyes Pigm 94:120–126
Haase C, Agner JA, Merkt F (2013) J Chem Phys 138:244202
Lacroix PG, Daran JC (1998) Chem Mater 10:1109–1114
Allen FH (2002) Acta Cryst B58:380–388
Thomas IR, Bruno IJ, Cole JC, Macrae CF, Pidcock E, Wood PA (2010) J Appl Cryst 43:362–366
APEX2 version 2.1-0, Bruker AXS Inc. Madison, (2004)
SAINT version 7.46a, Bruker AXS Inc. Madison, (2004)
Sheldrick GM (1996) SADABS. University of Göttingen, Göttingen
Sheldrick GM (2008) Acta Cryst A64:112–122
Brandenburg K (2012) DIAMOND version 3.2i, Crystal Impact GbR. Bonn, Germany
Spek AL (2009) Acta Cryst D65:148–155
Kuz’mina LG, Vedernikov AI, Vedernikov AI, Lobova NA, Sazonov SK, Basok SS, Howard JAK, Gromov SP (2009) Russ Chem Bull 58:1192–1210
Kuz’mina LG, Vedernikov AI, Sazonov SK, Lobova NA, Churakov AV, Lermontova EK, Howard JAK, Alfimov MV, Gromov SP (2011) Russ Chem Bull 60:1734–1761
Smith G, Wermuth UD, Young DJ, White JM (2008) Acta Cryst C64:o123–o127
Glidewell C, Low JN, Skakle JMS, Wardell JL (2003) Acta Cryst C59:o144–o146
Deng Y-H, Wang S-Y, Liu J, Yang Y-L, Zhang F, Ma H-W (2007) Acta Chim Sin 65:809–815
Chadwick K, Sadiq G, Davey RJ, Seaton CC, Pritchard RG, Parkin A (2009) Cryst Growth Des 9:1278–1279
Glidewell C, Low JN, Skakle JMS, Wardell JL (2003) Acta Cryst C59:o509–o511
Glidewell C, Low JN, Skakle JMS, Wardell JL (2005) Acta Cryst C61:o246–o248
Smith G, Wermuth UD, Young DJ, Healy PC (2005) Acta Cryst E61:o2008–o2011
Seaton CC, Chadwick K, Sadiq G, Guo K, Davey RJ (2010) Cryst Growth Des 10:726–733
Guo M-L (2005) Acta Cryst E61:o1728–o1730
Yang R, Shen X-Q, Mao H-Y, Zhang H-Y, Wu Q-A, Wang H, Hou H-W, Zhu Y (2006) Synth React Inorg Met-Org, Nano-Met Chem 36:617–620
Smith G, Wermuth UD, Healy PC (2007) Acta Cryst E63:o3527–o3528
Li Z-S, Chai J-S (2007) Acta Cryst E63:o2857–o2859
Deng Y-H, Liu J, Wu B, Ambrus C, Keene TD, Waldmann O, Liu S-X, Decurtins S, Yang X-J (2008) Eur J Inorg Chem 2008:1712–1718
Li G-L, Yin W-D, Liu G-Z, Ma L-F, Huang L-L, Li L, Wang L-Y (2014) Inorg Chem Commun 43:165–168
Berger WE (1940) Helvet Chim Acta 23:39–53
Martell AE, Smith RM (1976) Critical stability constants. Plenum Press, New York
Würthner F, Kaiser TE, Saha-Möller CR (2011) Angew Chem Int Ed 50:3376–3410
Kitajgorodskij A (1973) Molecular crystals and molecules. Academic Press, New York
Acknowledgements
T. M. K. would like to thank the Alexander von Humboldt Foundation. R. W. S. is thankful to Professor Christian W. Lehmann for his support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seidel, R.W., Goddard, R., Spiteller, M. et al. The 1:1 Proton-Transfer Compounds of N,N -dimethyl-4-[(E)-2-(4-pyridinyl)vinyl]aniline with 3-Nitrophthalic and Perchloric acids. J Chem Crystallogr 45, 86–93 (2015). https://doi.org/10.1007/s10870-015-0569-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-015-0569-z