Abstract
Four compounds, [(HL) 3+3 · (cba−)3·2H2O] (1, cba = o-chlorobenzoate), [(HL)+·(NSA−)] (2, NSA− = 5-nitrosalicylate), [(HL) 2+2 ·(3,5-dns2−)] (3, 3,5-dns2− = 3,5-dinitrosalicylate), and [(HL) 2+2 ·(nda2−)] (4, nda2− = 1,5-naphthalenedisulfonate) were obtained from self-assembly of the corresponding acids with the cyclohexylamine, and their structures were fully characterized. All four compounds are organic salts, with proton transfer occurring to the NH2 of the cyclohexylamine moiety. All structures adopted hetero supramolecular synthons. Compound 1 crystallizes in the triclinic, space group P-1, with a = 10.151(3) Å, b = 13.160(4) Å, c = 17.172(5) Å, α = 72.159(4)°, β = 79.546(4)°, γ = 78.285(4)°, V = 2120.5(11) Å3, Z = 2. Compound 2 crystallizes in the monoclinic, space group P2(1)/c, with a = 13.501(2) Å, b = 6.3144(15) Å, c = 19.601(3) Å, β = 123.900(13)°, V = 1386.9(4) Å3, Z = 4. Compound 3 crystallizes in the monoclinic, space group P2(1)/n, with a = 14.6439(11) Å, b = 6.9231(4) Å, c = 21.3012(18) Å, β = 96.4800(10)°, V = 2145.7(3) Å3, Z = 4. Compound 4 crystallizes in the monoclinic, space group P2(1)/n, with a = 5.3477(5) Å, b = 19.9238(16) Å, c = 11.1761(9) Å, β = 95.7230(10)°, V = 1184.84(17) Å3, Z = 2. Analysis of the crystal packing of 1–4 displays that there are extensive N–H···O hydrogen bonds between the acids and the cyclohexylamine in all of the salts. Except the classical hydrogen bonding interactions, the secondary propagating interactions also play significant roles in structure extension. The four salts displayed 2D/3D framework structures for the synergistic effect of the various noncovalent interactions.
Graphical Abstract
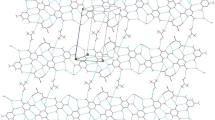
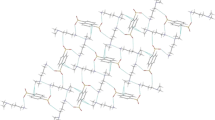
The crystal structures of the cyclohexylamine salts with o-chlorobenzoic acid, 5-nitrosalicylic acid, 3,5-dinitrosalicylic acid, and 1,5-naphthalenedisulfonic acid show extensive classical hydrogen bonding as well as C–H···O, CH2···O, Cl···O, NH3···π, CH2···π, and π···π interactions, giving 2D sheets or 3D networks.











Similar content being viewed by others
References
Janiak CJ (2000) J Chem Soc Dalton Trans 3885
Desiraju GR (2005) Chem Commun 2995
Lehn JM, Atwood JL, Davies JED, MacNicol DD, Vögtle F (eds) (1996) Comprehensive supramolecular chemistry. Pergamon, Oxford
Wood PA, Allen FH, Pidcock E (2009) CrystEngComm 11:1563
Veljković DŽ, Janjić GV, Zarić SD (2011) CrystEngComm 13:5005
Desiraju GR (2011) Cryst Growth Des 11:896
Burrows AD (2004) Struct Bond 108:55
Braga D, Maini L, Polito M, Grepioni F (2004) Struct Bond 111:1
Aakeröy CB, Beatty AM (2001) Aust J Chem 54:409
Etter MC (1990) Acc Chem Res 23:120
Holman KT, Pivovar AM, Swift JA, Ward MD (2001) Acc Chem Res 34:107
Gould PJ (1986) Int J Pharm 33:201
Shan N, Bond AD, Jones W (2002) Cryst Eng 5:9
Bhogala BR, Basavoju S, Nangia A (2005) CrystEngComm 7:551
Desiraju GR (1989) Crystal engineering: the design of organic solids. Elsevier, Amsterdam
Leiserowitz L (1976) Acta Crystallogr B32:775
Kolotuchin SV, Fenlon EE, Wilson SR, Loweth CJ, Zimmerman SC (1995) Angew Chem Int Ed Engl 34:2654
Kuduva SS, Craig DC, Nangia A, Desiraju GR (1999) J Am Chem Soc 121:1936
MacDonald JC, Dorrestein PC, Pilley MM (2001) Cryst Growth Des 1:29
Highfill ML, Chandrasekaran A, Lynch DE, Hamilton DG (2002) Cryst Growth Des 2:15
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556
Nichol GS, Clegg W (2009) Cryst Growth Des 9:1844
Men YB, Sun JL, Huang ZT, Zheng YQ (2009) CrystEngComm 11:978
Deng ZP, Huo LH, Zhao H, Gao S (2012) Cryst Growth Des 12:3342
Smith HW, Mastropaolo D, Camerman A, Camerman N (1994) J Chem Cryst 24:239
Wei XT, Li J, Yin HD (2011) Acta Cryst E67:o319
Wei B (2011) Acta Cryst E67:o2185
Jin SW, Zhang WB, Liu L, Gao HF, Wang DQ, Chen RP, Xu XL (2010) J Mol Struct 975:128
Jin SW, Zhang WB, Liu L, Wang DQ, He HD, Shi T, Lin F (2011) J Mol Struct 991:1
Jin SW, Wang DQ, Wang XL, Guo M, Zhao QJ (2008) J Inorg Organomet Polym 18:300
Wang DQ (2006) Acta Cryst E62:o2181
Bruker (2004) SMART and SAINT., Bruker AXS, Madison
Sheldrick GM (2000) SHELXTL, Structure Determination Software Suite, version 6.14. Bruker AXS, Madison
Lynch DE, Thomas LC, Smith G, Byriel KA, Kennard CHL (1998) Aust J Chem 51:867
Smith G, White JM (2001) Aust J Chem 54:97
Williams DH, Fleming I (1995) Spectroscopic methods in organic chemistry, 5th edn. McGraw-hill, London
Schultheiss N, Lorimer K, Wolfe S, Desper J (2010) CrystEngComm 12:742
Jin SW, Wang DQ, Xu YC (2012) J Coord Chem 65:1953
Bakshi PK, Linden A, Vincent BR, Roe SP, Adhikesavalu D, Cameron TS, Knop O (1994) Can J Chem 72:1273
Felloni M, Blake AJ, Hubberstey P, Wilson C, Schröer M (2002) CrystEngComm 4:483
Sundaralingam M, Jensen LH (1965) Acta Crystallogr 18:1053
Simith G, Hartono AW, Wermuth UD, Healy PC, White JM, Rae AD (2005) Aust J Chem 58:47
Smith G, Wermuth UD, Healy PC, White JM (2011) J Chem Crystallogr 41:1649
González FV, Jain A, Rodríguez S, Sáez JA, Vicent C, Peris G (2010) J Org Chem 75:5888
Etter MC, MacDonald JC, Bernstein J (1990) Acta Cryst B46:256
Gao XL, Lu LP, Zhu ML (2009) Acta Cryst C65:o123
Mitsui R, Nagasawa A, Watanabe S, Okamoto A, Yonezawa N (2010) Acta Cryst E66:o873
Acknowledgments
We gratefully acknowledge the financial support of the Education Office Foundation of Zhejiang Province (Project No. Y201017321) and the financial support of the Zhejiang A & F University Science Foundation (Project No. 2009FK63).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Lin, Z., Jin, S. et al. Crystal and Molecular Structure of Four Organic Salts Assembled from Cyclohexylamine and Organic acids. J Chem Crystallogr 44, 210–219 (2014). https://doi.org/10.1007/s10870-014-0503-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-014-0503-9