Abstract
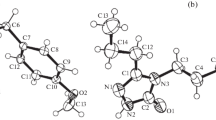
Derivatives hydrazone are attributed interesting pharmacological properties. The compound under study (E)-ethyl 4-(2-(thiofen-2-ylmethylene) hydrazinyl)benzoate (1), crystallized from ethanol as translucent light yellow thin plates. They are monoclinic, space group P21/c, with unit cell parameters a = 8.846(6), b = 20.734(14), c = 7.583(5) Å, β = 95.743(13)°, V = 1383.8(16) Å3. Three hydrogen bonds and two intermolecular π-interaction perpendiculars to the hydrogen bonds patterns are observed in the crystal structure. A detailed description of these interactions will be presented. Also, semiempirical calculations (PM6) show the formation in the gas phase of a supramolecular chain, emulating the C(8) graph set seen in the crystal structure of the compound.
Graphical Abstract
The synthesis and crystal structure of the title compound, C14H14N2O2S, is reported.






Similar content being viewed by others
References
Rollas S, Küçükgüzel G (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939
Abdel-Zaher E, Dib H, Al-Awadi N, Elnagdi M (2007) Chemistry of carbofunctionally substituted hydrazones. Arkivoc II:272–315
Belskaya N, Dehaen W, Bakulev V (2010) Synthesis and properties of hydrazones bearing amide, thioamide and amidine functions. Arkivoc I:275–332
Alvarado Y, Álvarez-Mon M, Baricelli J, Caldera-Luzardo J, Cubillán N, Ferrer-Amado G, Hassanhi M, Marrero-Ponce Y, Mancilla V, Rocafull M, San Antonio-Sánchez M, Ojeda-Andara J, Thomas L (2010) Solubility of thiophene-, furan- and pyrrole-2-carboxaldehyde phenylhydrazone derivatives in 2.82 mol L–1 aqueous DMSO at 298.15 K, inhibition of lymphoproliferation and tubulin polymerization: a study based on the scaled particle theory. J Sol Chem 39:1099–1112
Alvarado Y, Baricelli J, Caldera-Luzardo J, Cubillán N, Ferrer-Amado G, Marrero-Ponce Y, Mancilla V, Rocafull M, Ojeda-Andara J, Thomas L, Vera-Villalobos J, Morales-Toyo M (2011) Thermodynamics of solution, interaction with calf thymus DNA and anticancer activity of phenylhydrazone derivatives. J Sol Chem. 40(5):26–39
Alvarado Y, Caldera-Luzardo J, Ferrer-Amado G, Mancilla-Labarca V, Michelena E (2007) Determination of the apparent molar refraction and partial molar volume at infinite dilution of thiophene-, pyrrole- and furan-2-carboxaldehyde phenylhydrazone derivatives in acetonitrile at 293.15 K. J Sol Chem 36(6):1–11
Alvarado Y, Ballestas-Barrientos A, Cubillán N, Morales-Toyo M, Restrepo J, Ferrer-Amado G (2012) Peferential solvation of thiophene and furan-2-carboxaldehyde phenylhydrazone derivatives in DMSO-Water and DMSO-n-Octanol mixtures. Spectrochim Acta Part A 103:361–367
Bruker (1998b) SAINT: SAX Area-detector Integration, Version 5.01, Bruker AXS, Inc., Madison, Wisconsin, USA
Sheldrick G (2008) A short history of SHELX. Acta Cryst A 64:112–122
Hobza P (2011) The calculation of intermolecular interaction energies. Annu Rep Prog Chem Sect C 107:148–168
Stewart J (2007) Optimization of Parameters for Semiempirical Methods V: modification of NDDO Approximations and Application to 70 Elements. J Mol Modeling 13:1173–1213
Korth M, Pitoňák M, Rězácˇ J, Hobza P (2010) A transferable H-bonding correction for semiempirical quantum-chemical methods. J Chem Theory Comput 6:344–352
Karpfen A (2002) Cooperative effects in hydrogen bonding. Adv Chem Phys 123:469–510
Jurečka P, Černy J, Hobza P, Salahub D (2007) Density functional theory augmented with an empirical dispersion term. Interaction energies and geometries of 80 noncovalent complexes compared with ab initio quantum mechanics calculations. J Comput Chem 28(2):555–569
Karpfen A (1997) In: Scheiner S (ed) Molecular interacctions from.van der Waals to strongly bond molecular complexes. Wiley, Chichester, p 265
Allen F (2002) The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Cryst B 58:380–388
Kitamura T, Sato T, Mori M (2001) Unexpected results of enyne metathesis using a ruthenium complex containing an N-heterocyclic carbene ligand. Chem Commun 14:1258–1259
O’Malley SJ, Tan KL, Watzke A, Bergman RG, Ellman JA (2005) Total synthesis of (+)-Lithospermic acid by asymmetric intramolecular alkylation via catalytic C−H bond activation. J Am Chem Soc 127:13496–13497
Williams DE, Craycroft DJ (1987) Nonbonded hydrogen···hydrogen repulsion energy from ab initio SCF calculations of methane, ammonia, water, and methanol dimmers. J Phys Chem 91:6365–6373
Rincón L, Almeida R, García-Aldea D, Diez y Riega H (2001) Hydrogen bond cooperativity and electron delocalization in hydrogen fluoride clusters. J Chem Phys 114:5552–5562
Brandenburg K, Putz H (2005) DIAMOND. Crystal Impact GbR, Bonn
ACD/ChemSketch Freeware (2006) version 10.00, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com
Acknowledgments
The authors thank Fondo Nacional de Ciencia, Tecnología e Innovación (FONACIT Proyecto de apoyo a Grupos No. G-2005000403) and grant LOCTI-2007-0003, Instituto Zuliano de Investigaciones Tecnológicas (INZIT), CONDES-LUZ, and Petroregional del Lago S. A. (PERLA) for partial financial support of this work, and to Prof. Graciela Diaz de Delgado for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morales-Toyo, M., Alvarado, Y.J., Restrepo, J. et al. Synthesis, Crystal Structure Analysis, Small Cluster Geometries and Energy Study of (E)-Ethyl-4-(2-(thiofen-2-ylmethylene)hydrazinyl)benzoate. J Chem Crystallogr 43, 544–549 (2013). https://doi.org/10.1007/s10870-013-0455-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0455-5