Abstract
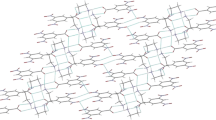
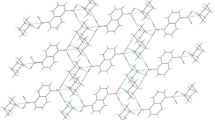
Two crystalline organic acid–base salts (nicotinamide):(3,5-dinitrosalicylic acid) [(HL+)···(3,5-dns−), 3,5-dns− = 3,5-dinitrosalicylate] (1), and (nicotinamide):(4-nitro-phthalic acid) [(HL+)···(Hnpa−), Hnpa− = 4-nitro-hydrogenphthalate] (2) derived from nicotinamide and aromatic carboxylic acids (3,5-dinitrosalicylic acid, and 4-nitro-phthalic acid) were prepared and characterized by X-ray diffraction analysis, IR, mp, and elemental analysis. Compound 1 crystallizes in the monoclinic, space group P2(1)/c, with a = 4.7950(3) Å, b = 22.290(2) Å, c = 14.3901(13) Å, β = 104.861(2)º, V = 1486.6(2) Å3, Z = 4. Compound 2 crystallizes in the monoclinic, space group P2(1)/c, with a = 15.0173(14) Å, b = 12.9849(13) Å, c = 7.7281(6) Å, β = 111.6040(10)º, V = 1401.1(2) Å3, Z = 4. Both supramolecular architectures of the compounds 1–2 involve O–H···O/N–H···O hydrogen bonds as well as other noncovalent association. The role of these noncovalent interactions in the crystal packing is ascertained. For the presence of these weak noncovalent interactions, both compounds displayed 3D framework structure.
Graphical Abstract
Due to the weak interactions, the compound displays 3D framework structure.







Similar content being viewed by others

References
Lam CK, Mak TCW (2000) Tetrahedron 56:6657
Tanase S, Bouwman E, Long GJ, Shahin AM, Mills AM, Jan Reedijk ALS (2004) Eur J Inorg Chem 4572
Janiak C (2000) J Chem Soc Dalton Trans 3885
Takahashi O, Kohno Y, Nishio M (2010) Chem Rev 110:6049
Berkovitch-Yellin Z, Leiserowitz L (1984) Acta Cryst B40:159
Cho KH, No KT, Scheraga HA (2000) J Phys Chem A 104:6505
Koch W, Frenking G, Gauss J, Cremer D (1986) J Am Chem Soc 108:5808
Desiraju GR (2002) Acc Chem Res 35:565
Braga D, Maini L, Paganelli F, Tagliavini E, Casolari S, Grepioni F (2001) J Organomet Chem 637–639:609
Liu JQ, Wang YY, Ma LF, Zhang WH, Zeng XR, Zhong F, Shi QZ, Peng SM (2008) Inorg Chim Acta 361:173
Biswas C, Drew MGB, Escudero D, Frontera A, Ghosh A (2009) Eur J Inorg Chem 2238
Maamen M, Gordon DM (1995) Acc Chem Res 28:37 and references therein
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ (2009) Cryst Growth Des 9:1106
Du M, Zhang ZH, Zhao XJ (2005) Cryst Growth Des 5:1247
Desiraju GR (1989) Crystal engineering, the design of organic solids. Elsevier, Amsterdam
Leiserowitz L (1976) Acta Crystallogr B32:775
Kolotuchin SV, Fenlon EE, Wilson SR, Loweth CJ, Zimmerman SC (1995) Angew Chem Int Ed Engl 34:2654
Kuduva SS, Craig DC, Nangia A, Desiraju GR (1999) J Am Chem Soc 121:1936
Bernstein J, Etter MC, Leiserowitz L (1994) Struct Correl 2:431
Moulton B, Zaworotko MJ (2001) Chem Rev 101:1629
Reddy LS, Bethune SJ, Kampf JW, Rodríguez-Hornedo N (2009) Cryst Growth Des 9:378
Lee IS, Shin DM, Chung YK (2003) Cryst Growth Des 3:521
Bhogala BR, Nangia A (2003) Cryst Growth Des 3:547
MacDonald JC, Dorrestein PC, Pilley MM (2001) Cryst Growth Des 1:29
Highfill ML, Chandrasekaran A, Lynch DE, Hamilton DG (2002) Cryst Growth Des 2:15
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556
Nichol GS, Clegg W (2009) Cryst Growth Des 9:1844
Men YB, Sun JL, Huang ZT, Zheng QY (2009) CrystEngComm 11:978
Báthori NB, Lemmerer A, Venter GA, Bourne SA, Caira MR (2011) Cryst Growth Des 11:75
Nicoli S, Bilzi S, Santi P, Caira MR, Li J, Bettini R (2008) J Pharm Sci 97:4830
Cheney ML, Shan N, Healey ER, Hanna M, Wojtas L, Zaworotko MJ, Sava V, Song S, Sanchez-Ramos JR (2010) Cryst Growth Des 10:394
Arenas-García JI, Herrera-Ruiz D, Mondragón-Vásquez K, Morales-Rojas H, Höpfl H (2010) Cryst Growth Des 10:3732
Athimoolam S, Natarajan S (2007) Acta Cryst E63:o1811
Athimoolam S, Natarajan S (2007) Acta Cryst E63:o2430
Koman M, Martiska L, Valigura D, Glowiak T (2003) Acta Cryst E59:o441
Zulfiya A, Zhao FH, Che YX (2010) Chin J Struct Chem 29:1185
Bruker (2004) SMART and SAINT. Bruker AXS, Madison, WI
Sheldrick GM (2000) SHELXTL, Structure Determination Software Suite, version 6.14. Bruker AXS, Madison, WI
Lynch DE, Thomas LC, Smith G, Byriel KA, Kennard CHL (1998) Aust J Chem 51:867
Smith G, White JM (2001) Aust J Chem 54:97
Smith G, Wermuth UD, Healy PC, White JM (2011) J Chem Crystallogr 41:1649
Aakeröy CB, Fasulo ME, Desper J (2007) Mol Pharm 4:317
Orola L, Veidis MV (2009) CrystEngComm 11:415
González FV, Jain A, Rodríguez S, Sáez JA, Vicent C, Peris G (2010) J Org Chem 75:5888
Ng SW, Naumov P, Drew MGB, Wojciechowski G, Brzezinski B (2001) J Mol Struct 595:29
Smith G, Wermuth UD, Bott RC, Healy PC, White JM (2002) Aust J Chem 55:349
Smith G, Lynch DE, Byriel KA, Kennard CHL (1995) Aust J Chem 48:1133
Borba A, Albrecht M, Gómez-Zavaglia A, Lapinski L, Nowak MJ, Suhm MA, Fausto R (2008) Phys Chem Chem Phys 10:7010
Acknowledgments
We gratefully acknowledge the financial support of the Education Office Foundation of Zhejiang Province (Project No. Y201017321) and the financial support of the Zhejiang A & F University Science Foundation (Project No. 2009FK63).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, S., Wang, D., Linhe, Q. et al. Crystal and Molecular Structure of Two Organic Acid–Base Salts from Nicotinamide and Aromatic Acids. J Chem Crystallogr 43, 258–265 (2013). https://doi.org/10.1007/s10870-013-0413-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0413-2