Abstract
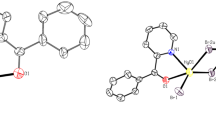
Mononuclear [Hg(L1)Br2] (1) and 1D polymer [Hg2(L2)Br4]n (2) [L1 = (N,N-diethyl,N′-(pyridin-2-yl)formylidene)ethane-1,2-diamine and L2 = (N,N-diethyl,N′-(pyridin-2-yl)benzylidene)ethane-1,2-diamine] have been synthesized using appropriate molar ratios of HgBr2 and L1/L2 in methanol–acetonitrile solution mixtures at room temperature. Crystal structures were investigated with the help of single crystal X-ray diffraction data. In 1, the mercury(II) center adopts a distorted square pyramidal geometry bound by three N atoms of L1 and two terminal bromides. Compound 2 forms a 1D chain through Hg–Br–Hg bridges in an infinite fashion connecting two crystallographically independent mercury(II) centers; Hg1 is in a distorted square pyramidal environment coordinated by three N atoms of L2 and two bridging bromides, whereas Hg2 adopts a tetrahedral geometry bound by two terminal and two bridging bromides. 1 and 2 display intraligand 1(π−π*) fluorescence in DMF solutions at room temperature.
Graphical Abstract







Similar content being viewed by others
References
Morsali A, Masoomi MY (2009) Coord Chem Rev 253:1882–1905
Nolte M, Pantenburg I, Meye G (2005) Z Anorg Allg Chem 631:2923–2927
Bharara MS, Parkin S, Atwood DA (2006) Inorg Chem 45:2112–2118
Wei KJ, Xie YS, Ni J, Zhang M, Liu QL (2006) Cryst Growth Des 6:1341–1350
Mahmoudi G, Morsali A, Hunter AD, Zeller M (2007) CrystEngComm 9:704–714
Hu C, Kalf I, Englert U (2007) CrystEngComm 9:603–610
Wang XF, Lv Y, Okamura Ta, Kawaguchi H, Wu G, Sun WY, Ueyama N (2007) Cryst Growth Des 7:1125–1133
Mahmoudi G, Morsali A (2008) Cryst Growth Des 8:391–394
Mahmoudi G, Morsali A (2008) Polyhedron 27:1070–1078
Zeng K-J, Xie Y-S, Ni J, Zhang M, Liu Q-L (2006) Cryst Growth Des 6:1341–1350
Yang J, Wu B, Zhuge F, Liang J, Jia C, Wang YY, Tang N, Yang XJ, Shi QZ (2010) Cryst Growth Des 10:2331–2341
Huber K (1997) In: Wisconsin mercury source book, Section 3, Madison, WI, pp. 67–677
Ko SK, Yang YK, Tac J, Shin I (2006) J Am Chem Soc 128:14150–14155
Tamayo A, Pedras B, Lodeiro C, Escriche L, Casabo J, Capelo JL, Covelo B, Kiveka R, Sillanpaa R (2007) Inorg Chem 46:7818–7826
Park S, Lee SY, Lee SS (2010) Inorg Chem 49:1238–1244
Petty M (2008) In: Molecular electronics: from principles to practice, Wiley, Chichester
Rosi NL, Kim J, Eddaoudi M, Chen B, O’Keeffe M, Yaghi OM (2005) J Am Chem Soc 127:1504–1518
MacGillivray LR, Papaefstathiou GS, Friscic T, Hamilton TD, Bucar DK, Chu Q, Varshney DB, Georgiev IG (2008) Acc Chem Res 41:280–291
Vigato PA, Tamburini S, Bertolo L (2007) Coord Chem Rev 251:1311–1492
Chattopadhyay S, Bhar K, Das S, Satapathi S, Fun HK, Mitra P, Ghosh BK (2010) Polyhedron 29:1667–1675
Satapathi S, Das S, Bhar K, Kumar RK, Maji TK, Ghosh BK (2011) Polyhedron 30:387–396
Rahaman SH, Chowdhury H, Bose D, Ghosh R, Hung CH, Ghosh BK (2005) Polyhedron 24:1755–1763
Englert U (2010) Coord Chem Rev 254:537–554
Yin Z, Wang W, Du M, Wang X, Gou J (2009) CrystEngComm 11:2441–2446
Notash B, Safari N, Khavasi HR (2010) Inorg Chem 49:11415–11420
Mercier N, Poiroux S, Riou A, Batail P (2004) Inorg Chem 43:8361–8366
Xu Z, Mitzi DB, Medeiros DR (2003) Inorg Chem 42:1400–1402
Steed JW, Atwood JL (2009) In: Supramolecular chemistry, 2nd edn. John Wiley, New York
SAINT Plus (1998), Data Reduction and Correction Program, v. 6.01, Bruker AXS, Madison
SADABS v.2.01 (1998), Bruker/Siemens area detector absorption correction program, Bruker AXS, Madison
Sheldrick GM (2008) Acta Crystallogr A64:112–122
Addision AW, Rao TN, Reedijik J, Rijn JV, Verschoor GC (1984) J Chem Soc, Dalton Trans 1349–1356
Nakamoto K (2009) In: Infrared and Raman spectra of inorganic and coordination compounds, Part B, 5th edn, John Wiley, New Jersey
Lever ABP (1984) In:Inorganic electronic spectroscopy, 2nd edn, Elsevier, New York
Wang X-F, Lv Y, Okamura T-a, Kawaguchi H, Wu G, Sun W-Y, Ueyama N (2007) Cryst Growth Des 7:1125–1133
Liu Y, Yan P-F, Yu Y-H, Hou G-F, Gao J-S, Lu JY (2010) Cryst Growth Des 10:1559–1568
Zeng F, Ni J, Wang Q, Ding Y, Ng SW, Zhu W, Xie Y (2010) Cryst Growth Des 10:1611–1622
Lakowicz JR (2006) In: Principles of fluorescence spectroscopy, 3rd edn, Springer, New York
Dutta B, Bag P, Florke U, Nag K (2005) Inorg Chem 44:147–157
Acknowledgments
BKG thanks the DST and CSIR, New Delhi, India for financial support. SS, SC, SD and KB are grateful to CSIR New Delhi, India for fellowships. The authors also acknowledge the use of DST-funded National Single Crystal X-ray Diffraction Facility at the Department of Inorganic Chemistry, IACS, Kolkata, India for crystallographic studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satapathi, S., Choubey, S., Das, S. et al. Syntheses, Structures and Properties of Two Luminous Mercury(II) Bromides Containing Tridentate N-Donor Schiff Bases: Control of Coordination Number and Nuclearity by Varying Ligand Matrices. J Chem Crystallogr 42, 1060–1066 (2012). https://doi.org/10.1007/s10870-012-0359-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0359-9