Abstract
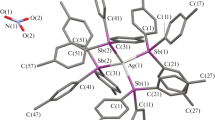
A novel silver-containing compound bis-benzylamino-silver(I) benzylcarbamate with the molecular formula of C22H26N3O2Ag and structural formula of C6H5CH2NHCOOAg(NH2CH2C6H5)2 is synthesized by the reaction of benzylamonium benzylcarbamate and silver oxide. It crystallizes in triclinic crystal system and P-1 space group with a = 5.2006(5) Å, b = 14.6298(15) Å, c = 14.7246(15) Å, α = 68.729(2)°, β = 83.507(2)°, γ = 85.412(2)°, and Z = 2. In the crystal, all of the silver atoms are two-coordinate, i.e., one silver atom symmetrically chelates with the two amino groups in two benzylamine molecules, while all the carboxylate groups only act as charge balancers. In addition, the crystal contains a large quantity of hydrogen bonds sustaining the stability of three-dimensional structure.
Index Abstract
A novel compound bis-benzylamino-silver(I) benzylcarbamate with a special molecular structure of two-coordinate is conveniently synthesized and it crystallizes in triclinic crystal system and P-1 space group with a = 5.2006(5) Å, b = 14.6298(15) Å, c = 14.7246(15) Å, α = 68.729(2)°, β = 83.507(2)°, γ = 85.412(2)°, and Z = 2.




Similar content being viewed by others
References
Zheng SL, Tong ML, Tan SD, Wang Y, Shi JX, Tong YX, Lee HK, Chen XM (2001) Organometallics 20:5319
Szlyk E, Piszczek P, Grodzicki A, Chaberski M, Golinski A, Szatkowski J, Blaszczyk T (2001) Chem Vap Depos 7:111
Chi KM, Chen KH, Peng SM, Lee GH (1996) Organometallics 15:2575
Chi KM, Lu YH (2001) Chem Vap Depos 7:116
Binnemans K, Van Deun R, Thijs Bm Vanwelkenhuysen I, Geuens I (2004) Chem Mater 16:2021
Jaber F, Charbonnier F, Faure R (1997) J Chem Crystallogr 27:397
Whitcomb DR, Rogers R (1996) J Chem Crystallogr 26:99
Chiong HA, Daugulis O (2006) Organometallics 25:4054
Ferguson G, McCrindle R, Parvez M (1984) Acta Crystallogr C40:354
Emeleus JC, Nyholm SR, Trotman-Dickenson AF (1973) Comprehensive inorganic chemistry, vol. 3. Pergamon Press Ltd., Oxford, p 79
Cotton FA, Wilkinson G (1962) Advanced inorganic chemistry. Wiley–Interscience, New York, p 862
Zheng GC, Kong MX, Shen ZJ (2006) Chinese Patent No CN 1767951A (in Chinese)
Liu JG, Cao Y, Li XY, Wang XY, Zeng XY (2010) Appl Phys A 100:1157
Teng KF, Vest RW (1988) IEEE Electron Device Lett 9:591
Jung HC, Cho SH, Joung JW, Oh YS (2007) J Electron Mater 36:1211
Kamyshny A, Ben-Moshe M, Aviezer S, Magdassi S (2005) Macromol Rapid Commun 26:281
George M, Weiss RG (2001) J Am Chem Soc 123:10393
Wright HB, Moore MB (1948) J Org Chem 70:3865
Katchalski E, Berliner-Klibanski C, Berger A (1951) J Org Chem 73:1829
Hoerr CW, Harwood HJ, Ralston AW (1944) J Org Chem 9:201
Sheldrick GM (1990) SHELXS-97, program for crystal structure solution. Göttingen University, Germany
Sheldrick GM (1997) SHELXL-97, program for crystal structure refinement. Göttingen University, Germany
Nao S, Kazunori I, Atsushi O, Hiroyuki H (2007) US Patent 7429341
Houng YC, Lin HC, Shih CJ, Shih SJ (2006) US Patent 7135394
Chun SK, Grudinin D, Lee DW, Kim SH, Yi GR, Hwang I (2009) Chem Mater 21:343
Alcock NW, Tracy VM, Waddington TC (1976) J Chem Soc Dalton Trans 5:2243
Jaber F, Charbonnier F, Faure R (1994) J Chem Crystallogr 24:681
Acknowledgments
This project is supported by the National Natural Science Foundation of China (Grant No. 51172081). We thank the Analytical and Testing Center of Huazhong University of Science and Technology for the test of MS, FT-IR, elemental analysis, TGA and DTA. We also thank Hua Li from College of Chemistry and Molecular Science of Wuhan University for collecting and solving the X-ray structures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y., Liu, JG., Li, XY. et al. Synthesis and Structure of Compound Bis-benzylamino-silver(I) Benzylcarbamate. J Chem Crystallogr 42, 891–896 (2012). https://doi.org/10.1007/s10870-012-0332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0332-7