Abstracts
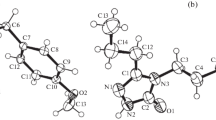
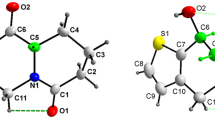
The 3-(propan-2-ylideneamino)-2,3-dihydro-2,2-dimethylquinazolin-4(1H)-one and 3-(3-nitrobenzylideneamino)-2,3-dihydro-2,2-dimethylquinazolin-4(1H)-one were synthesized and characterized by IR, 1H NMR and HRMS. The molecular structures were further confirmed by X-ray diffraction analysis. The former 1, C13H17N3O, is monoclinic, space group P2(1)/c, a = 9.056(2), b = 9.439(2), c = 14.872(3) Å, β = 133.121(12)°, Z = 4, V = 1270.9(5) Å3. The latter 2, C17H16N4O3, is also monoclinic, space group P2(1)/c, a = 10.0006(2), b = 12.2298(3), c = 13.3255(3) Å, β = 96.602(2)°, Z = 4, V = 1618.97(6) Å3. It is very interesting that the pyrimidine ring in the 1 adopts a half-chair confirmation, while adapting a skew-boat one in the structure of 2.
Graphical Abstract
The 3-(propan-2-ylideneamino)-2,3-dihydro-2,2-dimethylquinazolin-4(1H)-one and 3-(3-nitrobenzylideneamino)-2,3-dihydro-2,2-dimethylquinazolin-4(1H)-one were synthesized and characterized by IR, 1H NMR and HRMS. The molecular structures were further confirmed by X-ray diffraction analysis. The former 1, C13H17N3O, is monoclinic, space group P2(1)/c, a = 9.056(2), b = 9.439(2), c = 14.872 (3) Å, β = 133.121(12)°, Z = 4, V = 1270.9 (5) Å3. The latter 2, C17H16N4O3, is also monoclinic, space group P2(1)/c, a = 10.0006(2), b = 12.2298(3), c = 13.3255(3) Å, β = 96.602(2)°, Z = 4, V = 1618.97(6) Å3. It is very interesting that the pyrimidine ring in the 1 adopts a half-chair confirmation, while adapting a skew-boat one in the structure of 2.



Similar content being viewed by others
References
Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ (1994) Science 265:1093
Laszlo SE, Chang RS, Cheng TB, Faust KA, Greenlee WJ, Kivlighn SD, Lotti VJ, O’Malley SS, Schorn TW, Siegl PK, Tran J, Zingaro GJ (1995) Bioorg Med Chem Lett 5:1359
Johne S (1981) Pharmazie 36:583
Horton DA, Bourne GT, Smythe ML (2003) Chem Rev 103:893
Liu JF, Wilson CJ, Ye P, Sprague K, Sargent K, Si Y, Beletsky G, Yohannes D, Ng SC (2006) Bioorg Med Chem Lett 16:686
Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfieldt J (1988) J Med Chem 31:2235
Duarte CD, Barreiro EJ, Fraga CAM (2007) Mini-Rev Med Chem 7:1108
Alagarsamy V, Raja SV, Dhanabal K (2007) Bioorg Med Chem 15:235
Alagarsamy V, Pathak US (2007) Bioorg Med Chem 15:3457
Murugan V, Kulkarni M, Anand RM, Kumar EP, Suresh B, Reddy VM (2006) Asian J Chem 18:900
Godfrey AAA (2005) PCT Int Appl WO 2005012260 A2 10 Feb (2005) Chem Abstr 142:198095
Selvam P, Girija K, Nagarajan G, De Clerco E (2005) Indian J Pharm Sci 67:484
Alanine A, Gobbi LC, Kolczewski S, Luebbers T, Peters J-U, Steward L (2006) US Pat US 2006293350 A1 28 Dec (2006) Chem Abstr 146:100721
Chaturvedula PV, Chen L, Civiello R, Degnan AP, Dubowchik GM, Han X, Jiang XJ, Macor JE, Poindexter GS, Tora GO, Luo G (2007) US Pat US 2007149503 A1 28 Jun (2007) Chem Abstr 147:118256
Letourneau J, Riviello C, Ho K-K, Chan J-H, Ohlmeyer M, Jokiel P, Neagu I, Morphy JR, Napier SE (2006) PCT Int Appl WO 2006095014 A1 14 Sep (2006) Chem Abstr 145:315012
Sheldrick GM (2008) Acta Cryst A64:112
Acknowledgments
We are grateful to the National Natural Science foundation of China (20802061), a project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Qing Lan Project (08QLT001, 10QLD008) of Jiangsu Education Committee for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, J., Zhang, MM., Lu, L. et al. Diverse Confirmations in the Crystal Structures of 2,3-Dihydro-2,2-dimethylquinazolin-4(1H)-one Derivatives. J Chem Crystallogr 42, 701–705 (2012). https://doi.org/10.1007/s10870-012-0303-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0303-z