Abstract
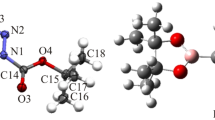
Two novel ferrocenyl substituted N-acetyl-2-pyrazolines, N-acetyl-3-(2-furyl)-5-ferrocenyl-2-pyrazoline (3) and N-acetyl-3-(2-thienyl)-5-ferrocenyl-2-pyrazoline (4), have been synthesized and characterized by FTIR, 1H-NMR, 13C-NMR techniques, elemental analysis and X-ray structure analysis. Thermal properties of these compounds have been determined by TGA, DTA and DSC analysis. Compound 3 (C19H18N2O2Fe) crystallizes in the monoclinic space group P21/c and Z = 4, with a = 8.6970(4) Å, b = 18.4725(9) Å, c = 11.0041(5) Å, β = 110.942(3)°. Compound 4 (C19H18N2OSFe) crystallizes in the orthorhombic space group Fdd2 and Z = 16, with a = 84.242(2) Å, b = 13.5416(5) Å, c = 5.9405(2) Å, β = 90°. In terms of crystal packing, each compound shows different molecular arrangement, which are stabilized by C–H···O intermolecular weak hydrogen bonds, and/or C–H···π interactions.
Index Abstract
Two novel ferrocenyl-containing 2-pyrazolines have been synthesized and fully characterized by FT-IR, 1H-NMR, 13C-NMR techniques, elemental analysis and X-ray crystal structure analysis. Thermal properties of these compounds have been determined by TGA, DTA and DSC analysis.









Similar content being viewed by others
References
Wiley RH, Behr LC, Fusco R, Jarboe CH (1967) In: Wiley RH (ed) The chemistry of heterocylic compounds. Interscience Publishers, New York
Pozharskii AF, Katritzky AR (2000) Handbook of heterocyclic chemistry. Elsevier, Oxford
Lévai A, Köver KE, Jeko J (2007) Arkıvoc viii:26
Budakoti A, Abid M, Azam A (2006) Eur J Med Chem 41:63
Lèvai A, Jeko J (2007) Arkıvoc i:134
Klimova EI, Lopez EAV, Klimova T, Garcia MM, Meleshonkova NN, Ramirez LR (2004) Russ J Gen Chem 1830:74
Özdemir A, Turan-Zitouni G, Kaplancıklı ZA, Revial G, Güven K (2007) Eur J Med Chem 42:403
Özdemir Z, Kandilci HB, Gümüşel B, Çalış Ü, Bilgin AA (2007) Eur J Med Chem 42:373
Jeong TS, Kim KS, Kim JR, Cho KH, Lee S (2004) Bioorg Med Chem Lett 14:2713
Csámpai A, Györfi AZ, Túrós GyI, Sohar P (2009) J Organomet Chem 694:3667
Johnson M, Younglove B, Lee L, LeBlanc R, Holt H, Hills P, Mackoy H, Brown T, Mooberry SL, Lee M (2007) Bioorg Med Chem 17:5897
Klimova EI, Garcia MM, Klimova T, Ramirez LR (2000) Russ Chem Bull 49:906
Hauser C, Pruett RL, Mashburn TA (1961) J Org Chem 26:1800
Berestneva TK, Garcia MM, Meleshonkova NN, Klimova EI (2001) Russ J Gen Chem 71:1626
Zora M, Görmen M (2007) J Organomet Chem 692:5026
Zora M, Velioğlu Ö (2008) J Organomet Chem 693:2159
Maity B, Roy M, Chakravarty AR (2008) J Organomet Chem 693:1395
Wu X, Wilairat P, Go ML (2002) Bioorg Med Chem Lett 12:2299
Delhaes L, Abessolo H, Biot C, Berry L, Delcourt P, Maciejewski L, Brocard J, Camus D, Dive D (2001) Parasitol Res 87:239
Domarle O, Blampain G, Agnanet H, Nzadiyabi T, Lebibi J, Brocard J, Maciejewski L, Biot C, Georges AJ, Millet P (1998) Antimicrob Agents Chemother 42:540
Top S, Tang J, Vessieres A, Carrez D, Provot C, Jaouen G (1996) Chem Commun 82:955
Top S, Dauer B, Vaissermann J, Jaouen G (1997) J Organomet Chem 541:355
Top S, Vessieres A, Cabestaing C, Laios I, Leclerq G, Provot C, Jaouen G (2001) J Organomet Chem 637:500
Top S, Vessieres A, Leclercq G, Quivy J, Tang J, Vaissermann J, Huche M, Jaouen G (2003) J Chem Eur 9:5223
Jaouen G, Top S, Vessieres A, Leclercq G, McGlinchey M (2004) J Curr Med Chem 11:2505
Kealy TJ, Pauson PL (1951) Nature 168:1039
Kelly PN, Preˆtre A, Devoy S, O’Rielly I, Devery R, Goel A, Gallagher JF, Lough AJ, Kenny PTM (2007) J Organomet Chem 692:1327
Fouda MFR, Abd-Elzaher MM, Abdelsamaia RA, Labib AA (2007) Appl Organomet Chem 21:613
Zsoldos-Mády V, Csámpai A, Szabó R, Mészáros-Alapi E, Pásztor J, Hudecz F, Sohár P (2006) ChemMedChem 1:1119
Fang J, Jin Z, Li Z, Liu W (2003) J Organomet Chem 674:1
Klimova T, Klimova EI, Martinez Garcia M, Vázquez López EA, Alvarez Toledano C, Toscano AR, Ruiz Ramirez L (2001) J Organomet Chem 628:107
Zora M, Pınar AN, Odabasoglu M, Büyükgüngör O, Turgut Cin G (2008) J Organomet Chem 693:145
López EAV, Klimova EI, Klimova T, Toledano CA, Ruíz Ramírez L, Toscano RA, Martínez García M (2004) Synthesis 15:2471
Lee KY, Kim JM, Kim N (1998) Tetrahedron Lett 39:3287
Fang J, Jin Z, Hu Y, Tao W, Shao L (2006) Appl Organomet Chem 20:813
Biot C, Glorian G, Maciejewski LA, Brocard JS (1997) J Med Chem 40:3715
Zora M, Tümay TA, Büyükgüngör O (2007) Tetrahedron 63:4018
Zora M, Kokturk M, Eralp T (2006) Tetrahedron 62:10344
Turgut Cin G, Demirel S, Çakıcı A (2011) J Organomet Chem 696:613
Liu J, Liu T, Dai H, Jin Z, Fang J (2006) Appl Organomet Chem 20:10
Wu X, Wilairat P, Go ML (2002) Bioorg Med Chem Lett 2299:12
Wu X, Tiekink ERT, Kostetski I, Kocherginsky N, Tan ALC, Khoo SB, Wilairat P, Go ML (2006) Eur J Pharm Sci 27:175
Ansari FL, Nazira S, Noureenb H, Mirzab B (2005) Chem Biodivers 2:1656
Sheldrick GM (1997) SHELXS-86 and SHELXL-97. Program for the refinement of crystal structures. University of Göttingen, Göttingen
STOE & CIE, X-AREA (Version 1.18) and X-RED32 (Version 1.04) (2002) Stoe & Cie, Darmstadt
Farrugia LJ, ORTEP-3 for Windows (1997) J Appl Cryst 30:565
Farrugia LJ (1999) J Appl Cryst 32:837
Turgut Cin G, Demirel S, Karadayı N, Büyükgüngör O (2008) a. Acta Cryst E m514:E64
Jian F, Zhao P, Guo H, Li Y (2008) Spectrochim Acta A 69:647
Kudar V, Zsoldos-Mády V, Simon K, Csámpai A, Sohár P (2005) J Organomet Chem 4018:690
Koksal Y, Isık S, Sahin G, Palaska E (2005) Acta Crystallogr C 61:0–542
Andrianov VG, Struchkov YuT, Postnov VN, Klimova EI, Sazonova VA (1984) J Organomet Chem 272:81
Shi Y-C, Zhu B-B, Sui C-X (2006) Acta Crystallogr A C62:m577
Erasmus JJC, Lamprecht GJ, Swarts JC, Roodt A, Oskarsson A (1996) Acta Crystallogr A C52:3000
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York
Yılmaz VT, Karadağ A, İçbudak H (1995) Thermochim Acta 261:107
Acknowledgments
The authors acknowledge the Research Board Akdeniz University (grand no BAP-2007.01.0105.001) for financial support, the Faculty of Arts and Sciences Ondokuz Mayıs University, for the Stoe IPDS-II diffractometer (purchased under grand F.279 of the University Research Fund) and the Faculty of Arts and Sciences Gaziosmanpasa University, for the Thermal and NMR analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cin, G.T., Demirel Topel, S., Cakıcı, A. et al. Synthesis, Crystal Structure and Thermal Properties of N-Acetyl-3-(2-furyl)-5-ferrocenyl-2-pyrazoline and N-Acetyl-3-(2-thienyl)-5-ferrocenyl-2-pyrazoline. J Chem Crystallogr 42, 372–380 (2012). https://doi.org/10.1007/s10870-011-0256-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0256-7