Abstract
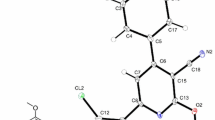
The crystal structure of the novel alcohol derivate from chlorthalidone, ethyl-chlorthalidone [2-chloro-5-(1-ethyl-3-oxo-1-isoindolinyl)benzenesulfonamide hydrated], C16H15ClN2O4S.H2O, was determined by X-ray diffraction at 295 K. This molecule crystallized in an orthorhombic space group Pca21 with a = 7.928(2) Å, b = 10.920(2) Å, c = 19.459(4) Å, α = β = γ = 90°. This compound is stabilized by intra and intermolecular NHO and OHO hydrogen bonds. These interactions which involve water molecules of the network give rise to a two-dimensional (2-D) array parallel to the (110) direction. The three-dimensional design of the compound is observed through weak NH···O hydrogen bonds (N2–H2A···O4). The NMR study was a complementary tool for crystal characterization. The spectra analysis clearly shows that signs of ethyl group atoms appear in strong field whereas the others atoms were identified in weak magnetic field (above of 140 ppm). The vibrational spectra present bands in the region of 2890–2940 cm−1 which can be assigned as symmetric and asymmetric modes of CH2 and CH3 groups. These results are in agreement with the crystal data which indicate the presence of ethyl group in this molecule.
Graphical Abstract
The crystal structure of the novel alcohol derivate from chlorthalidone (ethyl-chlorthalidone) is stabilized by intra and intermolecular NHO and OHO hydrogen bonds, that give rise to a two-dimensional (2-D) array parallel to the (110) direction.






Similar content being viewed by others
References
Martins FT, Bocelli MD, Bonfilio R, Araújo MB, Lima PV, Neves PP, Veloso MP, Ellena J, Doriguetto AC (2009) Cryst Growth Des 9:3235
Pandit N, Horhota ST (1990) Drugs in paths. US Patent. 4.933.360
Sheldrick GM (1997) SHELX97, Program for crystal structure refinement. University of Gottingen, Germany
Blessing RH (1995) Acta Crystallogr A 51:33
Farrugia L (1997) J Appl Crystallogr 30:565
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Van de Streek J (2006) J Appl Crystallogr 39:453
Graf W (1959) Helv Chim Acta 42:1085
Flack HD (1983) Acta Crystallogr A 39:876
Etter MC, MacDonald JC (1990) Acta Crystallogr B 46:256
Acknowledgments
The authors are thankful to the Brazilian Agencies CAPES, FAPEMIG, CNPq for the financial support, and LabCri (Departamento de Física–Universidade Federal de Minas Gerais) for X-ray facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, M.C., Franco, C.H.J., de Oliveira, V.E. et al. Crystal and Vibrational Analysis of a Novel Alcohol Derivate from Chlorthalidone: Ethyl-Chlorthalidone. J Chem Crystallogr 42, 232–237 (2012). https://doi.org/10.1007/s10870-011-0230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0230-4