Abstract
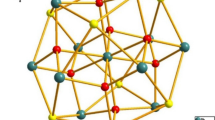
The reaction of propane-1,2-diamine (pdam) with lead (II) nitrate in the ethanol solution led to the formation of a lead (II) complex, [Pb(NO3)(pdam)2]NO3 (1), with interesting coordination environment of the lead (II) ion. The compound was characterized by elemental analysis and IR spectroscopy and its crystal structure was determined by single crystal X-ray structural analysis. Lead (II) ion is coordinated by two neutral N,N′-bidentate propane-1,2-diamine ligands and by an O,O′-bidentate nitrate ion in a complex cation of 1. There is also an uncoordinated nitrate ion as counter-ion. The primary coordination sphere around lead (II) ion includes four N atoms (Pb–N, 2.377(4)–2.644(4) Å). The effective coordination around lead (II) ion could be described as a highly distorted octahedron if the Pb···O contacts (Pb1···O4, 3.090(4) Å, Pb1···O5, 3.177(3) Å) are included. The coordination polyhedron is better described as a distorted pentagonal pyramid due to the presence of stereochemically active lead (II) ion lone pair of electrons in the axial position of the pyramid. Thus, the complex is hemidirected. Complex cations and nitrate counter-ions are connected into 3D structure by hydrogen bonds of the N–H···O type.
Graphical Abstract
Lead (II) complex with propane-1,2-diamine (pdam), [Pb (NO3) (pdam)2] NO3, was characterized by elemental analysis and IR spectroscopy and its crystal structure was determined by single crystal X-ray structural analysis.



Similar content being viewed by others
References
Casas JS, Sordo J (eds) (2006) Lead: chemistry, analytical aspects, environmental impact and health effects. Elsevier, Amsterdam
Dai J, Yang J, Li Y (2010) Acta Crystallogr E66:m298
Davidovich LR, Stavila V, Marinin DV, Voit EI, Whitmire KH (2009) Coord Chem Rev 253:1316
Shimoni-Livny L, Glusker PJ, Bock WC (1998) Inorg Chem 37:1853
Sun X, Tian X, Tomsig JL, Suszkiw JB (1999) Toxicol Appl Pharmacol 156:40
Chisholm JJ (1971) Sci Am 224:15
Allen FH (2002) Acta Crystallogr B58:380
Yang L-Q, Li X-H (2010) Acta Crystallogr E66:m310
Claudio ES, Goldwin HA, Magyar JS (2002) Prog Inorg Chem 51:1
Saxena G, Flora SJS (2004) J Biochem Mol Toxicol 18:221
Gracia RC, Snodgrass WR (2007) Am J Health Syst Pharm 64:45
Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM, Mijovilovich A, Parkin G, Zaleski JM, Penner-Hahn JE, Goldwin HA (2005) J Am Chem Soc 127:9495
Hettiarachchi GM, Pierzynski GM (2004) Environ Progr 23:78
Khatik SK, Thakur R, Sharma GD (2006) J Ind Pollut Control 22:233
Harrowfield JM, Miyamae H, Shand TM, Skelton BW, Soudi AA, White AH (1996) Aust J Chem 49:1043
Billing DG, Lemmerer A (2006) Acta Crystallogr E62:m1103
Oxford Diffraction (2009) CrysAlisPro, Version 171.33.53, Oxford Diffraction Ltd, Abingdon, Oxfordshire, England
Sheldrick GM (2008) Acta Crystallogr A64:112
Spek AL (2003) J Appl Crystallogr 36:7
Farrugia LJ (1997) J Appl Crystallogr 30:565
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood JPA (2008) J Appl Crystallogr 41:466
Gatehouse BM, Livingstone SE, Nyholm RS (1957) J Chem Soc 4222–4225
Addison CC, Gatehouse BM (1960) J Chem Soc 613–616
Ferraro JR (1959) J Inorg Nucl Chem 10:319
Williams NJ, Gan W, Reibenspies JH, Hancock RD (2009) Inorg Chem 48:1407
Mahjoub AR, Morsali A (2001) Z Kristallogr-NCS 216:601
Morsali A, Payeghader M, Monfared SS, Moradi M (2003) J Coord Chem 56:761
Abedini J, Morsali A, Zeller M (2008) Z Anorg Allg Chem 634:2659
Acknowledgments
This research was supported by Payame Noor University, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hakimi, M., Kukovec, BM., Schuh, E. et al. Preparation and Crystal Structure of Lead (II) Complex with Propane-1,2-Diamine. J Chem Crystallogr 42, 180–185 (2012). https://doi.org/10.1007/s10870-011-0221-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0221-5