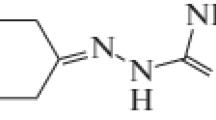Abstract
The structure of 5-N-hydroxyethanequindoline hydrochloride methanolate, C17H15ON2 Cl·½CH3OH, M r = 314.78, has been determined from X-ray diffraction data. The crystals are monoclinic, space group C2/c, with Z = 8 molecules per unit cell and a = 18.179(11), b = 7.317(5), c = 24.125(15) Å, β = 110.155(10)°, V c = 3012(3) Å3, crystal density D c = 1.388 Mg m−3. The structure was solved by direct methods, and the asymmetric unit comprises the 5-N-hydroxyethanequindoline hydrochloride and ½CH3OH moiety. The methanol is unusually disordered over a twofold axis with the C atom slightly removed from the twofold axis. Restraints were applied to the bond lengths of the two components of the disordered CH3OH, and to the anisotropic thermal displacement parameters of the disordered CH3OH carbon atom. The heterocyclic quindoline ring system and the first C atom of the hydroxyethane side chain are planar within 0.02 Å, with the terminal C–OH atoms of the side chain significantly out of the plane. The crystal structure is maintained via three hydrogen bonds all involving the chlorine atom an oxygen in the hydroxyethane side chain, a nitrogen in the quindoline moiety and the methanol oxygen.
Graphical Abstract
The novel cryptolepine analogue, 5-N-hydroxyethanequindoline was synthesised as part of an ongoing exploration of cryptolepine analogues as lead compounds towards antimalarial and anticancer agents. In previous work it has been shown that halogen ring substituents enhance the antimalarial properties of cryptolepine and improve its selectivity but the effects of modification of the 5-N substituent on antiplasmodial activity has not so far been investigated. Although, the antiplasmodial activity of this analogue was found to be less than that of cryptolepine, the X-ray structure of this compound will help to determine whether or not 5-N-hydroxyethanequindoline is able to intercalate into DNA and facilitate the design of new cryptolepine analogues with DNA binding properties appropriate for antimalarial (with no DNA intercalation) or anticancer (sequence-specific binding) applications.





Similar content being viewed by others
References
Wright CW, Addae-Kyereme J, Breen AG, Brown JE, Cox MF, Croft SL, Gokcek Y, Kendrick H, Phillips RM, Pollet PL (2001) J Med Chem 44:3187
Bonjean K, De Pauw-Gillet MC, Defrense MP, Colson P, Houssier C, Dassonneville L, Bailly C, Greimers R, Wright CW, Quentin-Leclercq J, Tits M, Angenot L (1998) Biochemistry 37:5136
Onyeibor O, Croft SL, Dodson HI, Feiz-Haddad M, Kendrick H, Millington NJ, Parapini S, Phillips RM, Seville S, Shnyder SD, Taramelli D, Wright CW (2005) J Med Chem 48:2701
Ablordeppy SY, Fan P, Clark AM, Nimrod A (1999) Bioorg Med Chem 7:343
Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J (2002) Nat Struct Biol 9:57
Bruker (2001) SMART (Version 5.625), SADABS (Version 2.03a) and SHELXTL (Version 6.12). Bruker AXS Inc., Madison
Bruker (2002) SAINT. Version 6.36a. Bruker AXS Inc., Madison
Cosier J, Glazer MJ (1986) J Appl Cryst 19:105
Sheldrick GM (1986) SHELX86, program for crystal structure determination. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL97, program for crystal structure refinement. University of Göttingen, Germany
Farrugia LJ (1999) J Appl Crystallogr 1999:837
Farrugia, L J (1997) In: Johnson CK and Burnett MN (eds) Based on ORTEP-III (v 1.0.3). J Appl Cryst 30:565
Merrit EA, Bacon DJ (1997) Raster 3D Graphics version 2.7c. Methods Enzymol 277:505–524. (Implemented in WinGX (qv) and generated by Ortep-3 for Windows)
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Cryst 41:466
Wright CW, Philipson JD, Lisgarten JN, Palmer RA (1999) J Chem Cryst 29:449
Lisgarten JN, Potter BS, Palmer RA, Pitts JE, Wright CW (2008) J Chem Cryst 38:807–813
Potter BS, Lisgarten JN, Palmer RA, Pitts JE, Wright CW (2008) J Chem Cryst 38:821–825
Sayle R (1994) RASMOL, a molecular visualisation program. Glaxo Research and Development, UK
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hampson, H.C., Ho, C.Y., Palmer, R.A. et al. Low Temperature X-Ray Crystallographic Structure of the Antiplasmodial Compound 5-N-Hydroxyethanequindoline Hydrochloride 0.5CH3OH. J Chem Crystallogr 41, 1757–1762 (2011). https://doi.org/10.1007/s10870-011-0169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0169-5