Abstract
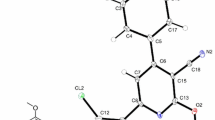
X-ray crystallographic data are provided for dichloroenol ethers (S,E)-2-(1-(1,2-dichlorovinyloxy)ethyl)-1,3,5-triisopropylbenzene (2) and (S,E)-2-(1-(1,2-dichloroprop-1-enyloxy)ethyl)-1,3,5-triisopropylbenzene (3). The former forms colorless crystals (orthorhombic, P212121 space group) and exhibits the following cell parameters: a = 10.212(5) Å; b = 10.359(8) Å; c = 18.217(6) Å. The latter also affords colorless crystals (monoclinic, P21 space group) with a = 13.558(2) Å; b = 10.891(1) Å; c = 15.260(2) Å; β = 115.65(1)°. The data complement those recently reported for two other dichloroenol ethers, ((1R,2S)-2-((E)-1,2-dichlorovinyloxy)cyclohexyl)benzene (5) and ((1R,2S)-2-((E)-1,2-dichloroprop-1-enyloxy)cyclohexyl)benzene (6). The X-ray analyses of these dichloroenol ethers, the only reported to date, establish unambiguously the trans stereochemistry of the chlorides in these and, by extension, similarly prepared enol ethers. This information was required for the complete mechanistic understanding of ynol ethers formation from dichloroenol ethers. Structural comparison of these dichloroenol ethers with some carbon (dichloroalkene) and nitrogen (dichloroenamine) analogues is also presented.
Graphical Abstract
This article reports the X-ray structures of four dichloroenol ethers, which establish the trans relationship of the chlorides. This determination of configuration is the starting point for the mechanistic elucidation of ynol ether formation from dichloroenol ethers.






Similar content being viewed by others
Notes
The structures of dichloroenol ethers 5 and 6, reported without detailed discussion in ref 3, represented the first examples.
References
Arens JF (1960) In: Raphael RA, Taylor EC, Wynberg H (eds) Advances in organic chemistry, vol II. Interscience Publishers Ltd, NY, pp 117–212
Rachenko SI, Petrov AA (1989) Russ Chem Rev 58:1671–1702
Normant JF (1963) Bull Soc Chim Fr 1876–1897
Moyano A, Charbonnier F, Greene AE (1987) J Org Chem 52:2919–2922
Kann N, Bernardes V, Greene AE (1997) Org Synth 14:13
Darses B, Milet A, Philouze C, Greene AE, Poisson J-F (2008) Org Lett 10:4445–4447
Montanari F, Negrini A (1957) Gazz Chim Ital 87:1061–1067
Montanari F, Negrini A (1957) Gazz Chim Ital 87:1068–1072
Ceccon J, Greene AE, Poisson J-F (2006) Org Lett 8:4739–4742
Fritsch P (1894) Liebigs Ann Chem 279:319
Buttenberg WP (1894) Liebigs Ann Chem 279:324
Wiechell H (1894) Liebigs Ann Chem 279:337
Knorr R (2004) Chem Rev 104:3795–3849
Eisler S, Tykwinski RR (2005) In: Diederich F, Stang PJ, Tykwinski RR (eds) Acetylene chemistry. chemistry, biology and material science, vol 7. Wiley-VCH, Weinheim
Flack HD (1983) Acta Crystallogr 39:876–881
Thompson AL, Watkin DJ (2009) Tetrahedron Asymmetr 20:712–717
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A (1993) J Appl Cryst 26:343
Molecular Structure Corporation TeXsan. Single Crystal Structure Analysis Software. Version 1.7. MSC, 3200 Research Forest Drive, The Woodlands, USA, 1992–1997
Johnson CK (1976) ORTEPII. Report ORNL-5138. Oak Ridge National Laboratotry, Oak Ridge
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc Perkin Trans II:S1
Johnson F (1968) Chem Rev 64:375–411
Hoffmann RW (1989) Chem Rev 89:1841–1860
Delair P, Brot E, Kanazawa A, Greene AE (1999) J Org Chem 64:1383–1386
Godage HY, Chambers DJ, Evans GR, Fairbanks AJ (2003) Org Biomol Chem 21:3772–3786
Jonczyk A, Michalski K (2002) Synlett 10:1703
Denonne F, Seiler P, Diederich F (2003) Helv Chim Acta 86:3096
Shimo T, Somekawa K, Wakikawa Y, Uemura H, Tsuge O, Imada K, Tanabe K (1987) Bull Chem Soc Jpn 60:621
Kende AS, Benechie M, Curran DP, Fludzinski P, Swenson W, Clardy J (1979) Tetrahedron Lett 20:4513
Acknowledgments
We thank Prof. P. Dumy (UJF) for his interest in our work and the French Ministry of Education, Research and Technology (MENRT) for a fellowship (to B.D.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darses, B., Philouze, C., Greene, A.E. et al. Dichloroenol Ethers X-ray Analysis in the Mechanistic Elucidation of Ynol Ethers Formation. J Chem Crystallogr 41, 1053–1059 (2011). https://doi.org/10.1007/s10870-011-0045-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0045-3