Abstract
The coordination polymer complex tetracesium bis(5-nitroisophthalate) heptahydrate [Cs4(C8H3NO6)2 (H2O)7] n has been synthesized and characterized using single-crystal X-ray diffraction. Crystals are monoclinic, space group P21/c, with Z = 4 in a cell with dimensions a = 12.3213(3), b = 6.7557(2), c = 36.2020(9) Å, β = 90.548(2)o. The complex is based on a repeating unit comprising four independent and different Cs coordination centres, two 6-coordinate, and two 8-coordinate [Cs–O, range 2.959(5)–3.386(5) Å], and seven water molecules, two of which are monodentate and the other five bridging, while all other oxygen atoms in the structure, including those of the nitro groups form inter-Cs bridges. Extensive water O–H···O hydrogen-bonding interactions give a three-dimensional framework. This structure represents the first of an alkali metal compound of 5-nitroisophthalic acid that has been reported.
Graphical Abstract
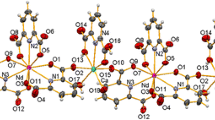
The coordination polymeric cesium complex with 5-nitroisophthalic acid (H2NIPA),[Cs4(NIPA)2 (H2O)7]n (1), has four separate and different coordination centres (two CsO6, two CsO8) linked through carboxyl, nitro and water oxygen bridges, giving a three-dimensional framework structure stabilized by hydrogen-bonding interactions.





Similar content being viewed by others
References
Guo J-Y, Zhang T-L, Liu Y-H, Yu K-B (2006) Hanneng Cailiao (Chin) 14:257
Chen H-J, Zhang J, Feng W-L, Fu M (2009) Inorg Chem Commun 9:300
Zhao N, Lian Z, Deng Y, Gu Y, Li X, Zhang J (2009) Z Kristallogr New Cryst Struct 224:323
Liu Y, He Q, Zhang X, Xue Z, Lv C (2009) Acta Crystallogr E65:m27
Lu H-J, Wang F-M (2009) Acta Crystallogr E65:m660
Ye M-D, Xiao H-P, Hu M-L (2004) Acta Crystallogr E60:m1516
Sun Y-X, Nie Y (2004) Acta Crystallogr E60:o1742
Smith G, Bott RC, Wermuth UD, Healy PC, White JM (2002) Aust J Chem 55:349
Smith G, Wermuth UD, Healy PC, White JM (2007) Aust J Chem 60:264
Aäkeroy CB, Hussain I, Forbes S, Desper J (2007) CrystEngComm 9:46
Chen D-M, Li X-H, Xiao H-P, Hu M-L (2005) Acta Crystallogr E61:o317
Rodriguez-Cuamatzi P, Arillo-Flores OI, Bernal-Uruchurti MI, Höpfl H (2006) Cryst Growth Des 5:167
Guo M-L (2004) Acta Crystallogr E60:1684
Okaya Y, Pepinsky R (1957) Acta Crystallogr 10:324
Teplova TB, Shibanova TA, Nolchanov VN, Okhrimenko TM, Belikova GS (1990) Kristallografiya 35:215
Pisareva AV, YuA Dubrovol’ski, Shilov GV, Karelin AI (2003) Electrokhim (Russian J. Electrochem.) 39:565
Luehrs DC, Bowman-James K (1994) J Mol Struct 321:251
Harnisch JA, Thomas LM, Guzei IA, Angelici RJ (1999) Inorg Chim Acta 286:207
Hu M, Geng C, Li S, Du Y, Jiang Y, Liu Z (2005) J Organomet Chem 690:3118
Smith G, Wermuth UD, Young DJ, White JM (2007) Polyhedron 26:3645
Oxford Diffraction (2008): CrysAlis CCD and CrysAlis RED, version 1.171.32.24. Oxford Diffraction Ltd., Yarnton, England
Sheldrick GM (1996) SADABS: absorption correction program for area detectors. University of Göttingen, Germany
Altomare A, Burla MC, Camalli M, Cascarno C, Giacovazzo A, Guagliardi A, Polidori G (1994) SIR92: structure solution program. J Appl Crystallogr 27:435
Sheldrick GM (2008) SHELXL97: structure refinement program. Acta Crystallogr A64:112
Farrugia LJ (1999) WinGX: a suite for small-molecule single-crystal crystallography. J Appl Crystallogr 32:837
Spek AL (2009) PLATON, a multipurpose crystallographic tool. Acta Crystallogr D65:148
Acknowledgments
The authors acknowledge financial support from the Australian Research Council and the Faculty of Science and Technology, Queensland University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, G., Wermuth, U.D. The Hydrogen-Bonded Coordination Polymer Tetracesium Bis(5-nitroisophthalate) Heptahydrate. J Chem Crystallogr 41, 688–692 (2011). https://doi.org/10.1007/s10870-010-9953-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9953-x