Abstract
The host compound 9,9′-(ethyne-1,2-diyl)-bis(fluoren-9-ol), H, forms inclusion compounds with caffeine (H·CAF) and methanol (H·MeOH). The host and guest ratios were 1:2 and 1:1, respectively. Both of these structures were successfully solved in P-1 with unit cell dimensions for H·CAF: a = 7.2121(14) Å, b = 9.2782(19) Å, c = 15.206(3) Å, α = 73.23(3)°, β = 84.20(3)°, γ = 73.39(3)°, Z = 2 and for H·MeOH : a = 9.7592(10) Å, b = 11.2584(11) Å, c = 20.7854(19) Å, α = 97.161(2)°, β = 99.263(2)°, γ = 95.257(2) Å, Z = 2. These crystal structures were studied together with that of a mixed clathrate, H·CAF·MeOH with stoichiometry 2:1:1.5. For H·CAF·MeOH which was solved in P21/c : a = 12.2051(5) Å, b = 46.8023(19) Å, c = 9.0121(4) Å, β = 91.9650(10)°, Z = 4. The kinetics of desolvation of the methanol solvate (H·MeOH) yielded an activation energy of 69.6–83.9 kJ mol−1.
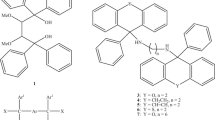
Graphical Abstract
The diol host 9,9′-(ethyne-1,2-diyl)-bis(fluoren-9-ol) forms an inclusion compound with caffeine and methanol.










Similar content being viewed by others
References
Mercer A, Trotter J (1978) Acta Crystallogr B34:450–453
Chandramohan A, Gayathri D, Velmurugan D, Ravikumar K, Kandhaswamy MA (2007) Acta Crystallogr E63:2495–2496
Trask AV, Motherwell WDS, Jones W (2005) Cryst Growth Des 5:1013–1021
Frišcic T, Trask AV, Jones W, Motherwell WD (2006) Angw Chem Int Ed 45:7546–7550
Gilli P, Pretto L, Bertolasi V, Gilli G (2009) Acc Chem Res 42:33–44
Bucar DK, Henry RF, Lou X, Duerst RW, Borchardt TB, MacGillivray LR, Zhang GGZ (2007) Mol Pharm 4:339–346
Danylyuk O, Suwinska K (2009) Chem Commun 5799–5813
Thuery P, Nierlich M, Asfari Z, Vicens J, Morikawa O, Konishi H (2001) Supramol Chem 13:521–527
Guo K, Sadiq G, Seaton C, Davey R, Yiu Q (2010) Cryst Growth Des 10:268–273
Segawa M, Mori K, Toda F (1988) Chem Lett 1755
Nassimbeni LR, Ramon G, Weber E (2007) J Therm Anal Calor 90:31–37
Le Roex T, Nassimbeni LR, Weber E (2007) Chem Commun 1124–1126
Weber E, Nitsche S, Wierig A, Csöregh I (2002) Eur J Org Chem 856
COLLECT (1998) Data Collection Software. Nonius, Delft
APEX 2 (2005) Version 1.0–27. Bruker AXS Inc, Madison
Otwinowski Z, Minor WZ (1997) In: Carter Jr CW, Sweet RM (ed) Methods in Enzymology. Macromolecular Crystallography, Part A, vol. 276. Academic Press, New York, pp 307–326
SAINT-Plus (2004) Version 7.12. Bruker AXS Inc, Madison
Sheldrick GM (1997) Shelx-97, Program for Crystal Structure Refinement. University of Göttingen, Germany
Barbour LJ (2001) X-Seed, a software tool for supramolecular crystallography. J Supramol Chem 1:189
Flynn JH, Wall LA (1966) J Polym Sci Part B: Polym Lett 4:323
Ozawa T (1965) Bull Chem Soc Jpn 38:1881
Barbour LJ (1999) Section, a computer program for the graphical display of cross sections through a unit cell. J Appl Cryst 32:353
Dean JA (1992) Lange’s handbook of chemistry, 14th edn. McGraw Hill, New York
Acknowledgments
We thank the CPUT and the NRF (Pretoria) for funding. CCDC 780740–780742 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by e-mailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, fax: +44(0)1223-336033.
Author information
Authors and Affiliations
Corresponding author
Additional information
The host compound 9,9′-(ethyne-1,2-diyl)-bis(fluoren-9-ol) forms inclusion compounds with caffeine (H·CAF) and methanol (H·MeOH). Both of these crystal structures were studied together with that of a mixed clathrate, H·CAF·MeOH. The kinetics of desolvation of the methanol solvate (H·MeOH) was determined.
Rights and permissions
About this article
Cite this article
Jacobs, A., Nassimbeni, L.R., Nohako, K.L. et al. Inclusion of Caffeine by a Diol Host. J Chem Crystallogr 41, 610–616 (2011). https://doi.org/10.1007/s10870-010-9933-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9933-1