Abstract
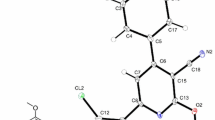
The title compound {6-[2-(2-chlorophenyl)-1,3-thiazol-4-yl]-2-oxo-1,3-benzothiazol-3(2H)-yl}acetic acid was prepared and characterized by elemental analyses, FT-IR, 1H NMR spectroscopy, X-ray diffraction. A quantum-chemical calculation was performed using the CNDO method. In the title compound, C18H11ClN2O3S2, the crystal structure is stabilized by intermolecular hydrogen bonds (C–H···O=C) to form centrosymmetric \( R_{2}^{2} \)(16) dimers and the C–H···O, O–H···N, and C–H···N interactions generating the graph set motifs \( R_{2}^{2} \)(9) and \( R_{2}^{2} \)(22).
Index Abstract

Synthetic route of title compound


Similar content being viewed by others
References
Fereira SH, Lorenzetti BB, Devissaguet M, Lesieur D, Tsouderos Y (1995) Br J Pharmacol 114:303
Ünlü S, Önkol T, Dündar Y, Ökçelik B, Küpeli E, Yeşilada E, Noyanalpan N, Şahin MF (2003) Arch Pharm Med Chem 336:353
Pavlović G, Soldin Ž, Popović Z, Tralić-Kulenović V (2007) Polyhedron 26:5162
Uzun L (2007) M.sc Thesis, Institute of Health Sciences, Gazi University
Enraf-Nonius (1994) CAD-4 express. Enraf-Nonius, Delft, The Netherlands
Harms K, Wocadlo S (1995) XCAD4. University of Marburg, Germany
Altomare A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A, Moliterni AGG, Polidor G, Spagna R (1999) J Appl Cryst 32:115
Sheldrick GM (2008) Acta Crystallogr A64:112
Farrugia LJ (1999) J Appl Cryst 32:837
North ACT, Phillips DC, Mathews FS (1968) Acta Crystallogr A24:351
Farrugia LJ (1997) J Appl Cryst 30:565
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc-Perkin Trans 2:1
Aydın A, Önkol T, Arıcı C, Akkurt M, Şahin MF, Ülkü D (2003) Acta Crystallogr E59:o616
Aydın A, Akkurt M, Önkol T, Büyükgüngör O (2006) Acta Crystallogr E62:o5933
Stout GH, Jensen LH (1968) X-ray crystal structure determination, a practical guide. Mac-Millan, New York, p 424
Etter MC (1990) Acc Chem Res 23:120
Bernstein J, Davis RE, Shimoni L, Chang N (1995) Angew Chem Int Ed Engl 34:1555
Pople JA, Beveridge DL (1970) Approximate molecular orbital theory. McGraw-Hill, New York
Acknowledgments
The authors wish to acknowledge the purchase of CAD4 diffractometer under grant DPT/TBAG1 of The Scientific and Technical Research Council of Turkey. In addition, this study is partly supported by a grant from Gazi University Research Fund (Project No: 02/2007-20).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aydın, A., Akkurt, M., Uzun, L. et al. Synthesis, Crystal Structure and Spectroscopic Characterization of {6-[2-(2-chlorophenyl)-1,3-thiazol-4-yl]-2-oxo-1,3-benzothiazol-3(2H)-yl}acetic acid. J Chem Crystallogr 40, 816–820 (2010). https://doi.org/10.1007/s10870-010-9744-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9744-4