Abstract
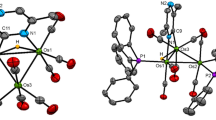
The reaction between the ethylidyne-substituted cluster MeCCo2MoCp(CO)8 (1) and the diphosphine ligand 2,3-bis(diphenylphosphino)maleic anhydride (bma) in refluxing CH2Cl2 has been investigated and found to afford the new mixed-metal clusters MeCCo2MoCp(CO)6[trans-2,3-bis(diphenylphosphino)succinic anhydride] (2) and Co2MoCp(CO)5[μ-C(Me)C=C(PPh2)C(O)OC(O)](μ-PPh2) (3), with the latter cluster representing the principal reaction product. Refluxing 1 with bma in either 1,2-dichloroethane or toluene yields only 3. The tetrahedrane cluster PhCCo2Mo(η5-C5H4CHO)(CO)8 (4), which contains a formyl-substituted cyclopentadienyl ring, has also been examined with added bma in refluxing CH2Cl2 and found to give only Co2Mo(η5-C5H4CHO)(CO)5[μ-C(Ph)C=C(PPh2)C(O)OC(O)](μ-PPh2) (5). All three products have been isolated and characterized spectroscopically in solution, and each molecular structure has been determined by X-ray crystallography. Cluster 2 contains a bridging diphosphine ligand with a succinic anhydride ring that results from the formal reduction of the maleic anhydride platform of the bma ligand, while clusters 3 and 5 each exhibit triangular Co2Mo cores, whose one face is capped by a 6e- C(R)C=C(PPh2)C(O)OC(O) [where R = Me (3), Ph (5)] ligand. The observed substitution products are discussed as a function of the capping carbyne group, ancillary polyene ligand, and related derivatives prepared by our groups.
Graphical Abstract
Thermolysis of the mixed-metal cluster MeCCo2MoCp(CO)8 (1) with bma in CH2Cl2 furnishes the new clusters MeCCo2MoCp(CO)6[trans-2,3-bis(diphenylphosphino)succinic anhydride] (2) and Co2MoCp(CO)5[μ-C(Me)C=C(PPh2)C(O)OC(O)](μ-PPh2) (3) as the minor and major products, respectively. The reaction between bma and the related benzylidyne-capped cluster PhCCo2Mo(η5-C5H4CHO)(CO)8 (4), which contains a formyl-substituted cyclopentadienyl ring, has also been examined and found to afford only Co2Mo(η5-C5H4CHO)(CO)5[μ-C(Ph)C=C(PPh2)C(O)OC(O)](μ-PPh2) (5) in moderate yield.





Similar content being viewed by others
References
Albano VG, Braga D, Ros R, Scrivanti A (1985) Chem Commun 866
Bruce MI, bin Shawkataly O, Snow MR, Tiekink ERT (1986) Aust J Chem 39:1109
Yang K, Bott SG, Richmond MG (1993) J Organomet Chem 454:273
Acum GA, Mays MJ, Raithby PR, Solan GA (1996) J Organomet Chem 508:137
Choi YY, Wong WT (1997) J Organomet Chem 542:121
Hui BKM, Wong WT (1998) J Chem Soc Dalton Trans 447
Bott SG, Yang K, Huang SH, Richmond MG (2004) J Chem Crystallogr 34:883
Watson WH, Wu G, Richmond MG (2005) Organometallics 24:5431
Richmond MG, Kochi JK (1987) Organometallics 6:254
Shiu KB, Peng SM, Cheng MC (1993) J Organomet Chem 453:133
Farrugia LJ, McDonald N, Peacock RD (1994) J Cluster Sci 5:341
Watson WH, Kandala S, Richmond MG (2005) J Chem Crystallogr 35:157
Watson WH, Kandala S, Richmond MG (2006) J Chem Crystallogr 36:813
Yang K, Smith JM, Bott SG, Richmond MG (1993) Organometallics 12:4779
Xia CG, Yang K, Bott SG, Richmond MG (1996) Organometallics 15:4480
Bott SG, Yang K, Talafuse KA, Richmond MG (2003) Organometallics 22:1383
Bott SG, Yang K, Richmond MG (2005) J Organomet Chem 690:3067
Bott SG, Yang K, Richmond MG (2006) J Organomet Chem 691:3771
Zhang W, Watson WH, Richmond MG (2008) J Chem Crystallogr 38:437
Seyferth D, Rudie CN, Merola JS (1978) J Organomet Chem 162:89
Nestle MO, Hallgren JE, Seyferth D (1980) Inorg Synth 20:226
Beurich H, Blumhofer R, Vahrenkamp H (1982) Chem Ber 115:2409
Blumhofer R, Fischer K, Vahrenkamp H (1986) Chem Ber 119:194
Jensen S, Robinson BH, Simpson J (1983) Chem Commun 1081
Wu HP, Yin YQ, Huang XY (1997) Inorg Chim Acta 255:167
Hart WP, Macomber DW, Rausch MD (1980) J Am Chem Soc 102:1196
Fenske D, Becher HJ (1975) Chem Ber 119:2115
Shriver DF (1969) The manipulation of air-sensitive compounds. McGraw-Hill, New York
SAINT Version 6.02, Bruker advanced analytical X-ray systems, Inc. Copyright 1997–1999
SHELXTL Version 5.1, Bruker advanced analytical X-ray systems, Inc. Copyright 1998
PLATON—a multipurpose crystallographic tool (2001) Spek AL, Utrecht University, Utrecht, The Netherlands
Coltrup NB, Daly LH, Wiberly SE (1990) Introduction to infrared and Raman spectroscopy. Academic Press, New York
Avey A, Schut DM, Weakley TJR, Tyler DR (1993) Inorg Chem 32:233
Shen H, Wang JC, Bott SG, Richmond MG (1997) J Chem Crystallogr 27:649
Watson WH, Chen T, Richmond MG (2004) J Chem Crystallogr 34:797
Mingos DMP, Wales DJ (1990) Introduction to cluster chemistry. Prentice-Hall, New Jersey
Beurich H, Vahrenkamp H (1982) Chem Ber 115:2385
Shimomura H, Lei X, Shang M, Fehlner TP (1997) Organometallics 16:5302
Zhang YH, Liu P, Xia CG, Hu B, Yin YQ (2003) J Organomet Chem 676:55
Song LC, Zhu WF, Hu QM, Wu H, Yu GA (2003) J Organomet Chem 667:143
Huang H, Hughes RP, Landis CR, Rheingold AL (2006) J Am Chem Soc 128:7454
Adams H, Guio LVY, Morris MJ, Spey SE (2002) J Chem Soc Dalton Trans 2907
Zhang J, Zhang YH, Chen XN, Ding ER, Yin YQ (2000) Organometallics 19:5032
Sutin KA, Li L, Frampton CS, Sayer BG, McGlinchey MJ (1991) Organometallics 10:2362
Lingham SL, Mays MJ, Raithby PR, Solan GA, Sundavadra BV, Conole G, Kessler M (1994) J Chem Soc Dalton Trans 3607
Fenske D, Bensmann W (1985) Z Naturforsch B Chem Sci 40:1093
Bott SG, Yang K, Richmond MG (2004) J Organomet Chem 689:791
Fenske D (1979) Chem Ber 112:363
Lewis JS, Heath SL, Powell AK, Zweit J, Blower PJ (1997) J Chem Soc Dalton Trans 855
Mao F, Philbin CE, Weakley TJR, Tyler DR (1990) Organometallics 9:1510
Yang K, Bott SG, Richmond MG (1995) Organometallics 14:2387
Orpen AG, Brammer L, Allen FH, Kennard O, Watson DG, Talyor R (1989) J Chem Soc Dalton Trans S1
Richter F, Beurich H, Muller M, Gartner N, Vahrenkamp H (1983) Chem Ber 116:3774
Kamiguchi S, Chihara T (2000) J Cluster Sci 11:483
Curtis MD, Han KR, Butler WM (1980) Inorg Chem 19:2096
Acknowledgments
Financial support from the Robert A. Welch Foundation (Grants P-0074-WHW and B-1093-MGR) is appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, W., Watson, W.H. & Richmond, M.G. Spectroscopic and X-ray Diffraction Data on Three Novel Trimetallic Clusters from Thermally Promoted Ligand Substitution in the Tetrahedrane Clusters MeCCo2MoCp(CO)8 and PhCCo2Mo(η5-C5H4CHO)(CO)8 . J Chem Crystallogr 39, 812–819 (2009). https://doi.org/10.1007/s10870-009-9573-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9573-5