Abstract
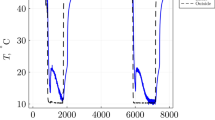
Investigation of the thermal properties of triphenylphosphonium p-toluenesulfonates, which are high temperature ionic liquids, reveals that they solidify to a noncrystalline material on fast cooling from the melt. During the study, a second crystalline form of [1-(methoxycarbonyl) ethyl] triphenylphosphonium p-toluenesulfonate (form II) has been obtained. Form II is monoclinic P21/c, with a = 11.002(5)Å, b = 12.793(5)Å, c = 18.805(5)Å, and β = 102.498(5)°. Comparison with form I reveals the existence of a common robust structural feature involving interactions between cations and anions.
Graphical Abstract
Investigation of thermal properties of ionic liquids has revealed a second crystalline form of [1-(methoxycarbonyl) ethyl] triphenylphosphonium p-toluenesulfonate.





Similar content being viewed by others
References
Wasserscheid P, Welton T (eds) (2003) Ionic liquids in synthesis. Wiley, Weinheim
Gordon CM (2001) Appl Catal A 222:101
Wasserscheid P, Keim W (2000) Angew Chem, Int Ed 39:3772. doi:10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5
Pernak J, Stefaniak F, Weglewski J (2005) Eur J Org Chem 650
Rickert PG, Antonio MR, Firestone MA, Kubatko K-A, Szreder T, Wishart JF, Dietz ML (2007) Dalton Trans 529
Bradaric CJ, Downard A, Kennedy C, Robertson AJ, Zhou Y (2003) Green Chem 5:143
Del Sesto RE, Corley C, Robertson A, Wilkes JS (2005) J Organomet Chem 690:2536
Cieniecka-Rosłonkiewicz A, Pernak J, Kubis-Feder J, Ramani A, Robertson AJ, Seddon KR (2005) Green Chem 7:855
Emnet C, Weber KM, Vidal JA, Consorti CS, Stuart AM, Gladysz JA (2006) Adv Synth Catal 348:1625
Domanska U, Casas LM (2007) J Phys Chem B 111:4109. doi:10.1021/jp070293j
Karodia N, Guise S, Newlands C, Andersen J-A (1998) Chem Commun 2341
Ludley P, Karodia N (2001) Tetrahedron Lett 42:2011
Comyns C, Karodia N, Zeler S, Andersen J-A (2000) Catal Lett 67:113. doi:10.1023/A:1019005202912
Bonnet LG, Kariuki BM (2006) Eur J Inorg Chem 437. doi:10.1002/ejic.200500769
Dupont J (2004) J Braz Chem Soc 15:341
Elaiwi A, Hitchcock PB, Seddon KR, Srinivasan N, Tan Y-M, Welton T, Zora JA (1995) J Chem Soc Dalton Trans 3467
van den Broeke J, Stam M, Lutz M, Kooijman H, Spek AL, Deelman B-J, van Koten G (2003) Eur J Inorg Chem 2798
Consorti CS, Suarez PAZ, de Souza RF, Burrow RA, Farrar DH, Lough AJ, Loh W, da Silva LHM, Dupont J (2005) J Phys Chem B 109:4341
Dupont J, Suarez PAZ, de Souza RF, Burrow RA, Kintzinger J-P (2000) Chem Eur J 6:2377. doi:10.1002/1521-3765(20000703)6:13<2377::AID-CHEM2377>3.0.CO;2-L
Sheldrick GM (1998) SHELX97 [Includes SHELXS97, SHELXL97]—programs for crystal structure analysis (Release 97-2); Institüt für Anorganische Chemie der Universität, Tammanstrasse 4, D-3400 Göttingen, Germany
Chen H, Kwait DC, Gonen ZS, Weslowski BT, Abdallah DJ, Weiss RG (2002) Chem Mater 14:4063. doi:10.1021/cm010930x
Desiraju GR (1995) Angew Chem Int Ed Engl 34:2311. doi:10.1002/anie.199523111
Scudder M, Dance I (2000) Dalton 2909
Dance I, Scudder M (1995) J Chem Soc Chem Commun 1039
Acknowledgments
The Universities of Birmingham and Cardiff are thanked for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kariuki, B.M., Bonnet, L.G. & Warren, J.E. High Temperature Ionic Liquids: Thermal Properties and Dimorphism in [1-(Methoxycarbonyl) Ethyl] Triphenylphosphonium p-Toluenesulfonate. J Chem Crystallogr 39, 693–697 (2009). https://doi.org/10.1007/s10870-009-9549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9549-5