Abstract
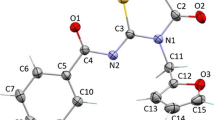
Two novel benzothiazoles 2-chloro-N-(benzothiazol-2-yl)benzamide (1) and 2-chloro-N-(6-cyanobenzothiazol-2-yl)benzamide (2) were obtained in multistep synthesis. They were characterised by means of IR, 1H- and 13C-NMR spectroscopy and also by single-crystal X-ray diffraction. The compound 1 crystallises with triclinic space group P \( \bar 1 \), a = 9.5923(8) Å, b = 9.8583(8) Å, c = 13.8962(10) Å, α = 89.162(6)°, β = 77.741(7)°, γ = 80.064(7)°, V = 1264.5(2) Å3, Z = 4 and compound 2 crystallises as methanol solvate with monoclinic space group P 21/n, a = 7.5093(9) Å, b = 13.0211(14) Å, c = 16.032(2) Å, β = 92.717(10)°, V = 1565.9(3) Å3, Z = 4. Both crystal structures consist of discrete dimers connected into a three-dimensional network by intermolecular C–H···O and C–H···X (X = Cl or S) hydrogen bonds and by face-to-face π–π stacking interactions.
Index Abstract
The synthesis and structure of two novel N-(benzothiazol-2-yl)benzamides. Irena Ćaleta, Dominik Cinčić, GraceKarminski-Zamola and Branko Kaitner. Hydrogen bonds and π–π interactions in N-(benzothiazol-2-yl)benzamides N-(benzothiazol-2-yl)benzamides.







Similar content being viewed by others
References
North M (1994) Contemp Org Synth I:475
Beckwith ALJ (ed) (1970) The chemistry of amides: synthesis of amides. Interscience, New York, p 96
Ban M, Taguchi H, Katsushima T, Takahashi M, Shinoda K, Watanabe A, Tominaga T (1998) Bioorg Med Chem 6:1069
Papadopoulou C, Geronikaki A, Hadjipavlou-Litina D (2005) Il Farmaco 60:969
Yoshida M, Hayakawa I, Hayashi N, Agatsuma T, Oda Y, Tanzawa F, Iwasaki S, Koyama K, Furukawa H, Kurakata S (2005) Bioorg Med Chem Lett 15:3328
Starčević K, Ćaleta I, Cinčić D, Kaitner B, Kralj M, Ester K, Karminski-Zamola G (2006) Heterocycles 68:2285
Baell JB, Forsyth SA, Gable RW, Norton RS, Mulder RJ (2002) J Comput Aided Mol Des 15:1119
(a) Das J, Moquin RV, Lin J, Liu C, Doweyko AM, DeFex HF, Fang Q, Pang S, Pitt S, Ren Shen D (2003) Bioorg Med Chem Lett 13:2587; (b) Das J, Lin J, Moquin RV, Shen Z, Spergel SH, Wityak J, Doweyko AM, DeFex HF, Fang Q, Pang S (2003) Bioorg Med Chem Lett 13:2145
Abdel-Aziz M, Matsuda K, Otsuka M, Uyeda M, Okawara T, Suzuki K (2004) Bioorg Med Chem Lett 14:1669
Sawhney SN, Boykin DW (1979) J Org Chem 44:1136
STARe Software V.9.01 (2006) Mettler–Toledo AG, Analytical, Schwerzenbach, Switzerland
Oxford Diffraction (2003) CrysAlis CCD and CrysAlis RED. Version 1.170. Oxford Diffraction Ltd, Wroclaw
Sheldrick GM (1998) SHELXS97, programs for crystal structure analysis. Institut für Anorganische Chemie der Universität, Göttingen
Sheldrick GM (1993) SHELXL93—program for crystal structure refinement. Institüt für Anorganische Chemie der Universität, Göttingen
Farrugia LJ (1999) Win GX J Appl Cryst 32:837
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG (1987) J Chem Soc Perkin Trans II S1
Farrugia LJ (1997) ORTEP-3 for Windows. J Appl Cryst 30:565
Mercury 1.4, Copyright CCDC 2001–2005, 12 Union Road, Cambridge
Hunter CA, Lawson KR, Perkins JC, Urch J (2001) J Chem Soc Perkin Trans 2:651
Acknowledgements
This research was supported by grants from the Ministry of Science and Technology of the Republic of Croatia (Grant Nos. 125-0982464-1356 and 119-1193079-3069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ćaleta, I., Cinčić, D., Karminski-Zamola, G. et al. The Synthesis and Structure of Two Novel N-(Benzothiazol-2-yl)benzamides. J Chem Crystallogr 38, 775–780 (2008). https://doi.org/10.1007/s10870-008-9389-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-008-9389-8