Abstract
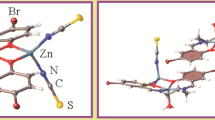
Three new complexes of zinc(II) with three different proton transfer compounds, obtained from pyridine-2,6-dicarboxylic acid (dipicolinic acid) and different Lewis bases, were synthesized and characterized using IR, 1H NMR and 13C NMR spectroscopy and single crystal X-ray diffraction. The chemical formulae and space groups of the complexes are (pipzH2)[Zn(pydc)2] · 4H2O, P21/n (1), (EDGnH2)[Zn(pydc)2] · 3H2O, P21/c (2) and (pdaH2)[Zn(pydc)2] · 4H2O, \( P\ifmmode\expandafter\bar\else\expandafter\=\fi{1} \) (3) where pydc, pipz, EDGn and pda are standing for dipicolinic acid, piperazine, ethylenediguanidine and 1,3-propanediamine respectively. Cell parameters of the complexes are a = 7.9493(4) Å, b = 13.4386(7) Å, c = 21.0557(11) Å, β = 90.415(5)° for 1; a = 9.785(3) Å, b = 25.671(4) Å, c = 9.3402(16) Å, β = 90.790(17)° for 2 and a = 8.411(5) Å, b = 11.650(7) Å, c = 12.793(8) Å, α = 115.534(9)°, β = 92.791(10)°, γ = 97.778(10)° for 3. The three crystal structures illustrate that the metal ion is six-coordinated by two pydc’s. In all three compounds a large number of O–H⋯O, N–H⋯O and C–H⋯O hydrogen bonds are observed. These interactions as well as other noncovalent interactions such as ion–pairing and π–π stacking play an important role in the formation and stabilization of supramolecular systems in the crystal lattices.
Index Abstract
The main purpose of this paper is to report and discuss about the synthesis, characterization, crystal structure and non-covalent interactions of three supramolecular frameworks of six-coordinated Zn(II) complexes, obtained by the reaction of different proton transfer compounds, i.e. (pipzH2)(pydc), (EDGnH2) (pydc) · 3H2O and (pdaH2)(pydc) · (pydcH2) · 2.5H2O with corresponding metallic salts.








Similar content being viewed by others
References
Lindoy LF, Atkinson IM (2000) Self assembly in supramolecular systems. Royal Society of Chemistry, R.S.C.
Lehn J-M, Atwood JL, Davies JED, MacNicol DD, Vögtle F (eds) (1996) Comprehensive supramolecular chemistry. Pergamon, Oxford, UK
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer-Verlag, Berlin
Lehn JM (2000) In:Steed JW, Atwood JL (eds) Supramolecular chemistry. Wiley, Chichester
Moghimi A, Alizadeh R, Shokrollahi A, Aghabozorg H, Shamsipur M, Shockravi A (2003) Inorg Chem 42:1616
Aghabozorg H, Ghadermazi M, Ramezanipour F (2006) Acta Cryst E62:o1143
Aghabozorg H, Nakhjavan B, Ghadermazi M, Ramezanipour F (2006) Acta Cryst E62:m2835
Aghabozorg H, Sadr-khanlou E (2007) Acta Cryst E63:m1753
Moghimi A, Sheshmani Sh, Shokrollahi A, Aghabozorg H, Shamsipur M, Kickelbick G, Aragoni MC, Lippolis V (2004) Z Anorg Allg Chem 630:617
Ranjbar M, Taghavipur M, Aghabozorg H, Moghimi A, Jalali F, Shamsipur M (2002) Polish J Chem 76:785
Aghabozorg H, Ghadermazi M, Manteghi F, Nakhjavan B (2006) Z Anorg Allg Chem 632:2058
Aghabozorg H, Mohammad Panah F, Sadr-khanlou E (2006) Acta Cryst E62:m2509
Moghimi A, Sharif MA, Shokrollahi A, Shamsipur M, Aghabozorg H (2005) Z Anorg Allg Chem 631:902
Moghimi A, Sheshmani Sh, Shokrollahi A, Shamsipur M, Kickelbick G, Aghabozorg H (2005) Z Anorg Allg Chem 631:160
Aghajani Z, Sharif MA, Aghabozorg H, Naderpour A (2006) Acta Cryst E62:m830
Aghabozorg H, Aghajani Z, Sharif MA (2006) Acta Cryst E62:m1930
Aghabozorg H, Ghasemikhah P, Ghadermazi M, Sheshmani Sh (2006) Acta Cryst E62:m2835
Aghabozorg H, Zabihi F, Ghadermazi M, Attar Gharamaleki J, Sheshmani Sh (2006) Acta Cryst E62:m2091
Aghabozorg H, Ghadermazi M, Attar Gharamaleki J (2006) Acta Cryst E62:o3174
Moghimi A, Aghabozorg H, Sheshmani Sh, Kickelbick G, Soleimannejad J (2006) Anal Sci 21:x141
SMART v. 5.059 (1999) Bruker molecular analysis research tool. Bruker AXS, Madison, Wisconsin, USA
Sheldrick GM (1998) SADABS, v. 2.01, Bruker/Siemens area detector absorption correction program. Bruker AXS, Madison, Wisconsin, USA
Arora KK, Pedireddi VR (2003) J Org Chem 68(24):9177
Vishweshwar P, Nangia A, Lynch VM (2001) Chem Commun 43:179
Acknowledgements
We are grateful to Tarbiat Moallem and Ilam Universities for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghabozorg, H., Ghadermazi, M., Zabihi, F. et al. Novel Complexes of Zinc(II) with Different Proton Transfer Ion Pairs Obtained from Dipicolinic Acid: Synthesis, Characterization and X-ray Crystal Structure. J Chem Crystallogr 38, 645–654 (2008). https://doi.org/10.1007/s10870-008-9363-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-008-9363-5