Abstract
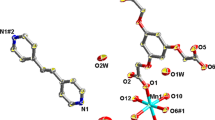
The self-assembly and structural characterization of the new manganese(II) [MnL 2](ClO4)2(CH3OH)0.5 (1) complex has been achieved. The crystallographic data for the complex 1: Orthorhombic Pbca , a = 16.287(2) Å, b = 21.293(2)Å, c = 32.718(3) Å, V = 11,347(2) Å3, Z = 8. The two polydentate ligands strand intertwined each other and around the manganese(II) ions forming a mononuclear complex. The Mn(II) ion is eight-coordinated with the eight donor nitrogen atoms of two ligands to form a distorted square antiprismatic coordination geometry. The magnetic properties investigation indicates that there is a weak antiferromagnetic exchange coupling between the Mn(II) ions of the complex.
Index Abstract
An octa-coordinated Mn(II) complex which exhibits a distorted square antiprismatic coordination geometry has been achieved by using a bisbidentate Schiff-base ligand.





Similar content being viewed by others
References
(a) Lehn JM (1995) Supramolecular chemistry, concepts and perspectives. VCH, Weinheim; (b) Atwood JL, Davies JED, MacNicol DD, Vogtle F, Lehn JM (eds) (1996) Comprehensive supramolecular chemistry. Pergamon, Oxford, 9:165
Schultheiss N, Powell DR, Bosch E (2003) Inorg Chem 42:8886
(a) Slagt VF, Kamer PCJ, van Leeuwen PWNM, Reek JNH (2004) J Am Chem Soc 126:1526; (b) Hogg L, Leigh DA, Lusby PJ, Morelli A, Parsons S, Wong JKY (2004) Angew Chem Int Ed 43:1218
(a) Bai Y, Guo D, Duan CY, Dang DB, Pang KL, Meng QJ (2004) Chem Commun 186; (b) Scarpellini M, Neves A, Hörner R, Bortoluzzi AJ, Szpoganics B, Zucco C, Silva RAN, Drago V, Mangrich AS, Ortiz WA, Passos WAC, de Oliveira MCB, Terenzi H (2003) Inorg Chem 42:8353
(a) Benny PB, Barnes CL, Piekarski PM, Lydon JD, Jurisson SS (2003) Inorg Chem 42:6519; (b) Arnold PL, Blake AJ, Wilson C, Love JB (2004) Inorg Chem 43:8206
(a) Shimazaki Y, Tani F, Fukui K, Naruta Y, Yamauchi O (2004) J Am Chem Soc 126:13236; (b) Wagler J, Doert T, Roewer G (2004) Angew Chem Int Ed 43:2441
(a) Nitschke JR (2004) Angew Chem Int Ed 43:3073; (b) Klein H-F, Camadanli S, Beck R, Leukel D, Flörke U (2005) Angew Chem Int Ed 44:975; (c) Oh M, Carpenter G, Sweigart DA (2004) Acc Chem Res 37:1
(a) Pal PK, Chowdhury S, Drew MGB, Datta D (2000) New J Chem 24:931; (b) Chowdhury S, Iveson PB, Drew MGB, Tocher DA, Datta D (2003) New J Chem 27:193
Lόpez-Torres E, Mendiola MA, Pastor CJ, Pérez BS (2004) Inorg Chem 43:5222
Halper SR, Cohen SM (2005) Inorg Chem 44:486
(a) Bretonnière Y, Mazzanti M, Pécaut J, Olmstead MM (2002) J Am Chem Soc 124:9012; (b) Gao EQ, Bai SQ, Yue YF, Wang ZM, Yan CH (2003) Inorg Chem 42:3642
(a) Vaira MD, Mani F, Stoppioni P (1992) J Chem Soc Dalton Trans 1127; (b) Bu XH, Chen W, Mu LJ, Zhang ZH, Zhang RH, Clifford T (2000) Polyhedron 2095
(a) Fang CJ, Duan CY, Guo D, He C, Meng QJ, Wang ZM, Yan CH (2001) Chem Commun 2540; (b) Guo D, Qian CQ, Duan CY, Pang KL, Meng QJ (2003) Inorg Chem 42:2024. (c) Bai Y, Dang DB, Duan CY, Song Y, Meng QJ (2005) Inorg Chem 44:5972
SMART and SAINT (1996) Area detector control and integration software. Siemens Analytical X-ray Systems, Inc., Madison
Sheldrick GM (1997) SHELXTL V5.1, software reference manual. Bruker, AXS, Inc., Madison
Bennett MV, Long JR (2003) J Am Chem Soc 125:2394
(a) Guo D, He C, Duan CY, Qian CQ, Meng QJ (2002) New J Chem 26:796; (b) Desiraju GR (1995) Angew Chem Int Ed 34:2311
Acknowledgment
We are grateful for financial support from the National Natural Science Foundation of China (No. 20701012), the Foundation of the Education Ministry of China (No. 207068) and the Education Department of Henan Province of China (No. 2007150003, 2007150011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dang, D., Bai, Y. & Duan, C. Crystal Structure and Magnetic Properties of a Novel Octa-coordinated Manganese(II) Complex. J Chem Crystallogr 38, 557–560 (2008). https://doi.org/10.1007/s10870-008-9335-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-008-9335-9