Abstract
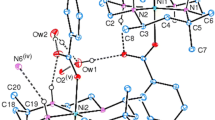
Two isostructural mononuclear compounds with formula {[Cd(dipm)(H2O)(ClO4)](dipm)(ClO4)(H2O)} (1) {[Zn(dipm)(H2O)(BF4)](dipm)(BF4)(H2O)} (2) and (in which dipm = bis(pyrimidin-2-yl)amine) have been synthesised and characterised by X-ray crystallography and infrared spectroscopy. The structure of compound 1 has been solved in the space group P21/n with a = 18.860(4), b = 8.579(2), c = 20.917(4) Å, β = 101.33(3)°, V = 3318.4(12) Å3, Z = 4 with final R = 0.0454. The structure of compound 2 has also been solved in the space group P21/n with a = 19.026(4), b = 8.389(1), c = 20.720(4) Å, β = 101.37(3)°, V = 3242.2(10) Å3, Z = 4 with final R = 0.0689. The geometry around the metal ions is octahedral, and is constituted by four nitrogen atoms from two dipm molecules, an oxygen atom from a water molecule and a semi-coordinating anion atom (\( O_{{\rm ClO}_{4}} \) for compound 1 and \( F_{{\rm BF}_{4}} \) for compound 2). In the lattice are also present: a non-coordinating water molecule, an anion molecule and a dipm molecule. For compound 1, the Cd–N distances are between 2.296 and 2.328 Å. The \( \hbox{Cd}{-}\hbox{O}_{{\rm H}_{2}{\rm O}} \) distance is 2.310 Å and the \( \hbox{Cd}{-}\hbox{O}_{{\rm ClO}_{4}} \) is 2.477 Å. The Zn–N distances in compound 2 are between 2.121 and 2.164 Å. The \( \hbox{Zn}{-}\hbox{O}_{{\rm H}_{2}{\rm O}} \) distance is 2.147 Å and the \( \hbox{Zn}{-}\hbox{F}_{{\rm BF}_{4}} \) distance is 2.373 Å. A hydrogen bond interaction of the Watson–Crick type is observed between the amine N atom of a dipm ligand to a pyrimidyl N atom and a non-coordinating dipm ligand with N···N distances, which vary from 3.066(5) to 3.109(5) Å. Furthermore medium to strong hydrogen bond interactions are present between oxygen atoms of the water molecules and the anions of the compounds.
Index Abstract
Two isostructural mononuclear compounds with formula {[Cd(dipm)(H2O)(ClO4)](dipm)(ClO4)(H2O)} (1) {[Zn(dipm)(H2O)(BF4)](dipm)(BF4)(H2O)} (2) and (in which dipm = bis(pyrimidin-2-yl)amine) have been synthesised and characterised by X-ray crystallography and infrared spectroscopy.

.




Similar content being viewed by others
References
Beatty AM (2001) Cryst Eng Comm 3:243 and references cited therein
Sundberg MR (2000) Rev Inorg Chem 20:95
Tadokoro M, Nakasuji K (2000) Coord Chem Rev 198:205
Sugiyama Y, Adachi K, Kawata S, Kumagai H, Inoue K, Katada M, Kitagawa S (2000) Cryst Eng Comm 2:174
Bishop MM, Lindoy LF, Skelton BW, White AH (2002) J Chem Soc Dalton Trans 377
Marshall SR, Incarvito CD, Shum WW, Rheingold AL, Miller JS (2002) Chem Comm 3006
Mohamadou A, van Albada GA, Kooijman H, Wieczorek B, Spek AL, Reedijk J (2003) New J Chem 27:983
Xie YS, Liu QL, Jiang H, Ni J (2003) Eur J Inorg Chem 22:4010
Rodríguez-Martín Y, Lorenzo Luis PA, Ruiz-Pérez C (2002) Inorg Chim Acta 328:169
Tadokoro M, Kanno H, Kitajima T, Shimada-Umemoto H, Nakanishi N, Isobe K, Nakasuji K (2002) PNAS 99:4950
Yao W, Kavallieratos K, de Gala S, Crabtree RH (2000) Inorg Chim Acta 311:45
van Albada GA, Mutikainen I, Turpeinen U, Reedijk J (2002) Acta Cryst C58:m179
Kooijman H, Spek AL, van Albada GA, Gamez P, Reedijk J (2004) Acta Cryst C60:m51
van Albada GA, Roubeau O, Gamez P, Kooijman H, Spek AL, Reedijk J (2004) Inorg Chim Acta 357:4522
Gamez P, van Albada GA, Mutikainen I, Turpeinen U, Reedijk J (2005) Inorg Chim Acta 358:1975
van Albada GA, Mutikainen I, Turpeinen U, Reedijk J (2006) Inorg Chem Commun 9:1067
van Albada GA, Mutikainen I, Turpeinen U, Reedijk J (2007) J Mol Struct 837:43
van Albada GA, van der Horst MG, Mutikainen I, Turpeinen U, Reedijk J (2007) Inorg Chem Commun 10:1014
van Albada GA, Quiroz-Castro ME, Mutikainen I, Turpeinen U, Reedijk J (2000) Inorg Chim Acta 298:221
van Albada GA, Smeets WJJ, Spek AL, Reedijk J (2000) J Chem Cryst 30:11
Kottke T, Stalke D (1993) J Appl Crystallogr 26:615
Nonius (2002) COLLECT. Nonius BV, Delft, The Netherlands
Sheldrick GM, SHELXS-97 (1997) Program for crystal structure determination. University of Göttingen, Germany
Sheldrick GM, SHELXL-97 (1997) Program for the refinement of crystal structures. University of Göttingen, Germany
Nakamoto K (1978) Infrared and Raman spectra of inorganic and coordination compounds, 3rd edn. Wiley, New York
Reedijk J (1970) Recl Trav Chim 89:605
Reedijk J (1971) Recl Trav Chim 90:117
Marcelis ATM, Erkelens C, Reedijk J (1984) Inorg Chim Acta 91:129
van Boom SSGE, Reedijk J (1993) J Chem Soc Chem Commun 1397
Nakasuji K, Tadokoro M, Toyoda J, Mitsumi M, Itoh T, Iijima K (1996) Mol Cryst Liq Cryst Sci Technol Sect A 285:241
Acknowledgement
The work described in the present paper has been supported by the Leiden University Study group WFMO (Werkgroep Fundamenteel MaterialenOnderzoek).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Albada, G.A., van der Horst, M.G., Mutikainen, I. et al. Two Mononuclear Compounds with bis(Pyrimidin-2-yl)amine as a Ligand and as a Hydrogen-bonded Lattice Molecule. Synthesis, Structure and Spectroscopy of {[M(dipm)(H2O)(A)](dipm)(A)(H2O)} (M = Cd with A = ClO4; and Zn with A = BF4). J Chem Crystallogr 38, 519–523 (2008). https://doi.org/10.1007/s10870-008-9333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-008-9333-y