Abstract
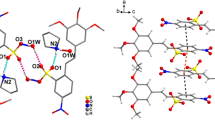
[1,2,3-13C3]-1-(Phenylsulfinyl)-3-benzyloxyacetone, C16H16O3S, (3) has been synthesized and its crystal structure has been determined by a single-crystal X-ray diffraction analysis. The X-ray diffraction study revealed that compound 3 crystallizes in the monoclinic crystal system in the acentric space group Pc, with cell constants at T = 100 K: a = 16.073(5), b = 5.5079(16), c = 7.949(2) Å, β = 100.221(4)°, V = 692.6(3) Å3, Z = 2, d calc = 1.383 g/cm3. Compound 3 contains the chiral tetravalent three-coordinated sulfur atom, which has a distorted tetrahedral configuration with a lone electron pair occupying one of the tetrahedron vertices. In the crystal, the molecules are packed in stacks along the b axis; the stacks consist of the molecules of the same chirality. Furthermore, the stacks of the molecules of the opposite chirality alternate along the c axis. The molecules in neighboring stacks are arranged by head-to-tail orientations. There are no short intermolecular contacts in the crystal of 3.



Similar content being viewed by others
References
(a) Solladie G, DemaillyG, Greck C (1985) J Org Chem 50:1552–1554; (b) Solladie G, Demailly G, Greck C (1985) Tetrahedron Lett 26:435–438; (c) Solladie G, Hutt J (1987) Tetrahedron Lett 28:797–800; (d) SolladieG, Colobert F (1996) J Org Chem 61:4369–4373; (e) Solladie G, Colobert F, Denni D, (1998) Tetrahedron: Asymmetry 9:3081–3094; (f) Solladie G, Hanquet G, Rolland C (1999) Tetrahedron Lett 40:177–180; (g) Solladie G, Hanquet G, Izzo I, Crumbie R (1999) Tetrahedron Lett 40:3071–3074
Wenzel SC, Williamson RM, Grunanger C, Xu J, Gerth K, Martinez RA, Moss SJ, Carroll BJ, Grond S, Unkefer CJ, Muller R, Floss HG (2006) J Am Chem Soc 128:14325–14336
(a) Hill A, Harris AP (1998) J Chem Soc Chem Commun 2361–2362; (b) Gerth K, Pradella S, Perlova O, Beyer S, Muller R (2003) J Biotechnol 106:233–253
Omura S, Tsuzuki K, Nakagawa A, Lukacs G (1983) J Anitibiot 36:611–613
Bindseil KU, Zeeck A (1994) Liebegs Ann Chem 305–312
Russell GA, Mikol GJ (1966) J Am Chem Soc 88:5498–5504
Rebiere R, Samuel O, Ricard L, Kagan HB (1991) J Org Chem 56:5991–5999
Nishide K, Nakyama A, Kusumoto T, Hiyama T, Takehara S, Shoji T, Osawa M, Kuriyama T, Nakamura K, Fujisawa T (1990) Chem Lett 623–625
Sheldrick GM (2003) SADABS, v. 2.03, bruker/siemens area detector absorption correction program. Bruker AXS, Madison, Wisconsin, USA
Sheldrick GM (2001) SHELXTL, v. 6.12, structure determination software suite. Bruker AXS, Madison, Wisconsin, USA
Solladie G (1981) Synthesis 3:185–196
Zhao SH, Samuel O, Kagan HB (1987) Tetrahedron 43:5135–5144
Cambridge structure database system, release (2006) Cambridge
Dahlen B (1973) Acta Crystallogr Sect B 29:595–602
Dahlen B (1974) Acta Crystallogr Sect B 30:642–646
Groth P (1985) Acta Chem Scand Ser A 39:587–591
Ferchaux Y, Villain F, Navaza A (1990) Acta Crystallogr Sect C 46:346–348
Yakovenko AA, Gallegos JB, Long RD, Timofeeva TV, Yu M (2006) Antipin Acta Crystallogr Sect E 62:o2484–o2486
Acknowledgments
Authors are grateful to the National Science Foundation for support via New Mexico EPSCoR program and NSF/DMR grant for acquisition of single-crystal X-ray diffractometer. VNK, RAM and TVT are grateful for National Institute of Health for support via NMHU RIMI program.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Asani, E.M., Khrustalev, V.N., Williamson, R.M. et al. Stereoselective Synthesis and X-Ray Structure Determination of Labeled [1, 2, 3-13C3]-1-(Phenylsulfinyl)-3-Benzyloxyacetone. J Chem Crystallogr 37, 663–667 (2007). https://doi.org/10.1007/s10870-007-9226-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-007-9226-5