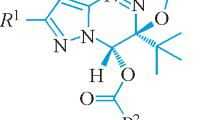
A new series of bis-triazenes, the 1-aryl-2-[3-(3-[2-aryl-1-diazenyl]-1,3-diazepan-1-ylmethyl)-1,3-diazepan-1-yl]-1-diazenes has been synthesized from the reaction of diazonium salts with a mixture of 1,4-diaminobutane and formaldehyde. The structures of 1-(p-bromophenyl)-2-[3-{3-[2-(p-bromophenyl)-1-diazenyl]-1,3-diazepan-1-ylmethyl}-1,3-diazepan-1-yl]-1-diazene(1), 1-(p-cyanophenyl)-2-[3-{3-[2-(p-cyanophenyl)-1-diazenyl]-1,3-di azepan-1-ylmethyl}-1,3-diazepan-1-yl]-1-diazene(2), and 1-(p-methoxyphenyl)-2-[3-{3-[2-(p-methoxy-phenyl)-1-diazenyl]-1,3-diazepan-1-ylmethyl}-1,3-diazepan-1-yl]-1 diazene(3) have been unequivocally determined by X-ray crystallography. The new bis-triazenes are important since the structure contains the novel saturated heterocycle, 1,3-diazepane. The general conclusion of this study is that alkanediamines with 3 or 4 carbon atoms in the spacer link between the nitrogen atoms give rise to the linear bicyclic molecules of type 5, in contrast to the case of ethylenediamine (spacer link 2 carbon atoms), which affords molecules of type 6, which exemplify the general cage structure of type 4. The crystal structures of 1, 2 and 3 are compared with the previously reported structure of the hexahydropyrimidine analogue 8a(X=CN); compounds 2 and 8a(X=CN) are homologous with respect to the alkane spacer moiety. The structures of 2 and 8a(X=CN) are very different in one respect; in 2 the aryldiazenyl-1,3-diazepanyl groups are in the s-trans orientation around the central methylene group whereas in 8a(X=CN) the arrangement of the aryldiazenyl-hexahydropyrimidinyl groups is the s-cis orientation.
Crystal data: 1 C23H30N8Br2, triclinic, space group P-1, a=8.3979(2), b=10.7828(3), c=14.4692(5) Å, α=83.670(1), β=78.662(1), γ=78.758(1)°, V=1256.48(6) Å3, for Z=2. 2 C25H30N10, monoclinic, space group P2 1 /n, a=13.4046(6), b=9.4482(4), c=10.6913(4)Å, β=103.239(2)°, V=2490.5(2) Å3, for Z=4. 3 C25H36N8O2, triclinic, space group P-1, a=8.5223(3), b=10.6913(4), c=14.4034(7)Å, α=85.657(2), β=78.731(2), γ=80.153(1)°, V=1266.88(9) Å3, for Z=2.




Similar content being viewed by others
References
Vaughan, K. Org. Preparat. Procedures Int. 2001, 33, 59.
Peori, M.B.; Vaughan, K; Hooper, D.L. J Org. Chem. 1998, 63, 7437.
Singer, R.D.; Vaughan, K.; Hooper, D.L. Can. J. Chem. 1986, 64, 1567.
Moser, S.L.; Church, R.; Peori, M.B.; Vaughan, K. Can. J. Chem. 2005, 83, 1071.
Peori, M.B.; Vaughan, K.; Bertolasi, V. J. Chem. Cryst. 2005, 35, 297.
Moser, S.L.; Bertolasi, V.; Vaughan, K. J. Chem. Cryst. 2005, 35, 307.
Tingley, R.; Peori, M.B.; Church, R.; Vaughan, K. Can. J. Chem. 2005, 83, 1799.
Tingley, R.; Bertolasi, V.; Vaughan, K. J. Chem. Cryst. 2005, 35, 821.
Vaughan, K.; Moser, S.L.; Tingley, R.; Peori, M.B.; Bertolasi, V. Can. J. Chem. 2006, 84, in press, November issue, available on line at http://canjchem.nrc.ca (doi: 10.1139/V06-091).
Glister, J.F.; Vaughan, K. J. Heterocycl. Chem. 2006, 43, 217.
Glister, J.F.; Vaughan, K.; Bertolasi, V. J. Chem. Cryst. 2004, 34, 175.
Glister, J.F.; Jenkins, H.; Vaughan, K. J. Chem. Cryst. 2006, 36, 445–452.
Otwinowski, Z.; Minor, W. In Methods in Enzymology, Vol. 276; Part A Carter, C.W.; Sweet, R.M., Eds.; Academic Press: London, 1997, pp. 307–326.
Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, C.; Moliterni, A.G.; Polidori, G.; Spagna, R. J. Appl. Crystallogr. 1999, 32, 115.
Sheldrick, G.M. SHELXL-97, Program for Refinement of Crystal Structures, University of Göttingen, Germany, 1997.
Nardelli, M. J. Appl. Crystallogr. 1995, 28, 659.
Burnett, M.N.; Johnson, C.K. ORTEP-III, Report ORNL-6895 Oak Ridge National Laboratory, Oak Ridge, TN, 1996.
Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, G.; Orpen, G.; Taylor, R. J. Chem. Soc. Perkin Trans. 2, 1987, S1.
Acknowledgements
The financial support (to KV) of the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant is gratefully acknowledged. The summer studentship of Reid Tingley, which made the synthetic work possible, was jointly funded by NSERC and a Research Grant provided by the Faculty of Graduate Studies & Research of Saint Mary’s University. This work was supported by the University of Ferrara.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
Crystallographic data (excluding structure factors) have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC 602053 (1), 602054 (2) and 602055 (3) . These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html or on application to CCDC, Union Road, Cambridge CB2 1EZ, UK [fax: (+44)1223-336033, e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Tingley, R., Bertolasi, V. & Vaughan, K. X-Ray crystal structure determination of a series of 1-aryl- 2-[3-(3-[2-aryl-1-diazenyl]-1,3-diazepan-1-ylmethyl)-1, 3-diazepan-1-yl]-1-diazenes obtained from the reaction of diazonium salts with mixtures of formaldehyde and 1,4-diaminobutane. J Chem Crystallogr 36, 831–839 (2006). https://doi.org/10.1007/s10870-006-9119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-006-9119-z