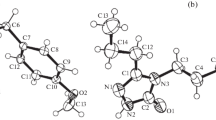
The molecular and crystal structures of the title compound, C19H22N2O4S, were determined by single crystal X-ray diffraction technique. The title compound crystallizes in space group F d d 2, with a = 30.785(3) Å, b = 10.6455(9) Å, c = 11.0036(8) Å, Z = 8, D calc = 1.379(2) g cm−3, μ(Mo-Kα) = 0.207 mm−1, and its crystal system is orthorhombic. The structure was solved by direct methods and refined to a final R = 0.042 for 1530 reflections with I > 2σ (I). There is a half-independent molecule in the asymmetric unit. The title molecule has twofold rotational symmetry along with the C–S bond. Classically no hydrogen bond is found in the crystal structure. The crystal structure is stabilized by π–π stacking and edge to face (C–H…π-ring) interactions. To elucidate conformational features and steric hindrances of the title molecule, selected torsion angle is varied from −180° to +180° in every 10° and thus molecular energy profile is calculated by PM3 semi-empirical method.




Similar content being viewed by others
References
Gök, Y.; Çetinkaya, E. Turkish J. Chem. 2004, 28, 157.
Günay, M.E.; Çetinkaya, B. XX. International Organometallic Chemistry Conference, 2002, Corfu, Greece, pp 223.
Günay, M.E. Ph.D. Thesis, 2004, Ege University, İzmir, Turkey.
Stoe & Cie, X-AREA (Version 1.18) and X-RED32 (Version 1.04), Darmstadt, Germany, 2002.
Sheldrick, G.M. SHELXS 97 and SHELXL 97, Program for Crystal Structure Solution and Refinement; University of Göttingen: Germany, 1997.
Stewart, J.J.P. J. Comput. Chem. 1989, 10, 209.
Stewart, J.J.P. J. Comput. Chem. 1989, 10, 221.
Roothaan, C.C. J. Rev. Mod. Phys. 1951, 23, 69.
Shchepin, R.; Litvinov, D. WinMopac 7.21 Semiempirical calculations program; Perm State University, Russia, 1998.
Farrugia, L.J. ORTEP-III for Windows, Department of Chemistry, University of Glasgow, UK, 1998.
Karabıyık, H.; Kılınçarslan, R.; Aygün, M.; Çetinkaya, B.; Büyükgüngör, O. Z. Naturforshung B 2005, 60, 837.
McGuinness, D.S.; Green, M.J.; Cavell, K.J.; Skelton, B.W.; White, A.H. J. Organomet. Chem. 1998, 565, 165.
Herrmann, W.A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G.R. J. Angew. Chem. Int. Engl. 1995, 34, 2371.
Herrmann, W.A.; Goossen, L.J.; Spiegler, M. J. Organomet. Chem. 1997, 547, 357.
Herrmann, W.A. Angew. Chem. Int. Ed. 2002, 41, 290.
Weskamp, T.; Böhm, V.P.W.; Herrmann, W.A.. J. Organomet. Chem. 2002, 12, 600.
Acknowledgments
Dokuz Eylül University Fund is gratefully acknowledged for its financial support (Project No: 04.KB.FEN.100), additionally Hasan Karabıyık would like to thank TÜBİTAK (The Scientific and Technical Research Council of Turkey) for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary materials
CCDC 261789 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: $+$44 1223 336033.
Rights and permissions
About this article
Cite this article
Karabıyık, H., Günay, M.E., Aygün, M. et al. Crystallographic and conformational analysis of 1,3-bis(2,4-dimethoxyphenyl)imidazolidine-2-thione. J Chem Crystallogr 36, 243–248 (2006). https://doi.org/10.1007/s10870-005-9005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-9005-0