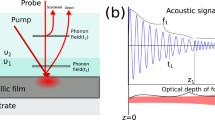
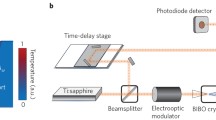
Abstract
An analytical model is presented for light scattering associated with heat transport near a cell membrane that divides a complex system into two topologically distinct half-spaces. Our analysis is motivated by experiments on vibrational photothermal microscopy which have not only demonstrated remarkably high contrast and resolution, but also are capable of providing label-free local information of heat transport in complex morphologies. In the first Born approximation, the derived Green’s function leads to the reconstruction of a full 3D image with photothermal contrast obtained using both amplitude and phase detection of periodic excitations. We show that important fundamental parameters including the Kapitza length and Kapitza resistance can be derived from experiments. Our goal is to spur additional experimental studies with high-frequency modulation and heterodyne detection in order to make contact with recent theoretical molecular dynamics calculations of thermal transport properties in membrane systems.



Similar content being viewed by others
Data availability
Data available on request.
References
Bertolotti, M., Li Voti, R.: A note on the history of photoacoustic, thermal lensing, and photothermal deflection techniques. J. App. Phys. 128(23), 230901 (2020)
Berciaud, S., Lasne, D., Blab, G.A., Cognet, L., Lounis, B.: Photothermal heterodyne imaging of individual metallic nanoparticles: theory versus experiment. Phys. Rev. B 73(4), 045424 (2006)
Berciaud, S., Cognet, L., Blab, G.A., Lounis, B.: Photothermal heterodyne imaging of individual nonfluorescent nanoclusters and nanocrystals. Phys. Rev. Lett. 93(25), 257402 (2004)
Adhikari, S., Spaeth, P., Kar, A., Baaske, M.D., Khatua, S., Orrit, M.: Photothermal microscopy: imaging the optical absorption of single nanoparticles and single molecules. ACS Nano 14(12), 16414–16445 (2020)
Gaiduk, A., Yorulmaz, M., Ruijgrok, P.V., Orrit, M.: Room-temperature detection of a single molecule’s absorption by photothermal contrast. Science 330(6002), 353–356 (2010)
Mertiri, A., Altug, H., Hong, M.K., Mehta, P., Mertz, J., Ziegler, L.D., Erramilli, S.: Nonlinear midinfrared photothermal spectroscopy using Zharov splitting and quantum cascade lasers. ACS Photonics 1(8), 696–702 (2014)
Totachawattana, A., Hong, M.K., Erramilli, S., Sander, M.Y.: Multiple bifurcations with signal enhancement in nonlinear mid-infrared thermal lens spectroscopy. Analyst 142(11), 1882–1890 (2017)
Pavlovetc, I.M., Podshivaylov, E.A., Chatterjee, R., Hartland, G.V., Frantsuzov, P.A., Kuno, M.: Infrared photothermal heterodyne imaging: contrast mechanism and detection limits. J. Appl. Phys. 127(16), 165101 (2020)
Zhang, D., Li, C., Zhang, C., Slipchenko, M.N., Eakins, G., Cheng, J.-X.: Depth-resolved mid-infrared photothermal imaging of living cells and organisms with submicrometer spatial resolution. Sci. Adv. 2(9), e1600521 (2016)
Li, Z., Aleshire, K., Kuno, M., Hartland, G.V.: Super-resolution far-field infrared imaging by photothermal heterodyne imaging. J. Phys. Chem. B 121(37), 8838–8846 (2017)
Aleshire, K., Pavlovetc, I.M., Collette, R., Kong, X.-T., Rack, P.D., Zhang, S., Masiello, D.J., Camden, J.P., Hartland, G.V., Kuno, M.: Far-field midinfrared superresolution imaging and spectroscopy of single high aspect ratio gold nanowires. Proc. Natl. Acad. Sci. U.S.A. 117(5), 2288–2293 (2020)
Zhang, Y., Yurdakul, C., Devaux, A.J., Wang, L., Xu, X.G., Connor, J.H., Ünlü, M.S., Cheng, J.-X.: Vibrational spectroscopic detection of a single virus by mid-infrared photothermal microscopy. Anal. Chem. 93(8), 4100–4107 (2021)
Zong, H., Yurdakul, C., Bai, Y., Zhang, M., Ünlü, M.S., Cheng, J.-X.: Background-suppressed high-throughput mid-infrared photothermal microscopy via pupil engineering. ACS Photonics 8(11), 3323–3336 (2021)
Bai, Y., Zhang, D., Lan, L., Huang, Y., Maize, K., Shakouri, A., Cheng, J.X.: Ultrafast chemical imaging by widefield photothermal sensing of infrared absorption. Sci. Adv. 5(7), eaav7127 (2019)
Paiva, E.M., Schmidt, F.M.: Ultrafast widefield mid-infrared photothermal heterodyne imaging. Anal. Chem. 94(41), 14242–14250 (2022)
Tamamitsu, M., Toda, K., Shimada, H., Honda, T., Takarada, M., Okabe, K., Nagashima, Y., Horisaki, R., Ideguchi, T.: Label-free biochemical quantitative phase imaging with mid-infrared photothermal effect. Optica. 7(4), 359–366 (2020)
Robert, H.M.L., Holanová, K., Bujak, Ł, Vala, M., Henrichs, V., Lánský, Z., Piliarik, M.: Fast photothermal spatial light modulation for quantitative phase imaging at the nanoscale. Nat. Commun. 12(1), 2921 (2021)
Zhang, Y., Zong, H., Zong, C., Tan, Y., Zhang, M., Zhan, Y., Cheng, J.-X.: Fluorescence-detected mid-infrared photothermal microscopy. J. Am. Chem. Soc. 143(30), 11490–11499 (2021)
Yin, J., Lan, L., Zhang, Y., Ni, H., Tan, Y., Zhang, M., Bai, Y., Cheng, J.-X.: Nanosecond-resolution photothermal dynamic imaging via MHZ digitization and match filtering. Nat. Commun. 12(1), 7097 (2021)
Zharov, V.P.: Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nat. Photon. 5(2), 110–116 (2011)
Samolis, P.D., Sander, M.Y.: Phase-sensitive lock-in detection for high-contrast mid-infrared photothermal imaging with sub-diffraction limited resolution. Opt. Express 27(3), 2643–2655 (2019)
Samolis, P.D., Langley, D., O’Reilly, B.M., Oo, Z., Hilzenrat, G., Erramilli, S., Sgro, A.E., McArthur, S., Sander, M.Y.: Label-free imaging of fibroblast membrane interfaces and protein signatures with vibrational infrared photothermal and phase signals. Biomed. Opt. Exp. 12(1), 303–319 (2021)
Chen, K., Song, B., Ravichandran, N.K., Zheng, Q., Chen, X., Lee, H., Sun, H., Li, S., Udalamatta Gamage, G.A.G., Tian, F., Ding, Z.: Ultrahigh thermal conductivity in isotope-enriched cubic boron nitride. Science. 367(6477), 555–559 (2020)
Legrand, R., Abi Ghanem, M., Plawinski, L., Durrieu, M.C., Audoin, B., Dehoux, T.: Thermal microscopy of single biological cells. Appl. Phys. Lett. 107(26), 263703 (2015)
Baffou, G., Rigneault, H., Marguet, D., Jullien, L.: A critique of methods for temperature imaging in single cells. Nat. Methods 11(9), 899–901 (2014)
Sotoma, S., Zhong, C., Kah, J.C.Y., Yamashita, H., Plakhotnik, T., Harada, Y., Suzuki, M.: In situ measurements of intracellular thermal conductivity using heater-thermometer hybrid diamond nanosensors. Sci. Adv. 7(3), eabd7888 (2021)
Okabe, K., Uchiyama, S.: Intracellular thermometry uncovers spontaneous thermogenesis and associated thermal signaling. Commun. Biol. 4(1), 1–7 (2021)
Okabe, K., Inada, N., Gota, C., Harada, Y., Funatsu, T., Uchiyama, S.: Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 3(1), 705 (2012)
Garner, A.L., Deminsky, M., Bogdan Neculaes, V., Chashihin, V., Knizhnik, A., Potapkin, B.: Cell membrane thermal gradients induced by electromagnetic fields. J. App. Phys. 113(21), 214701 (2013)
Nakano, T., Kikugawa, G., Ohara, T.: A molecular dynamics study on heat conduction characteristics in DPPC lipid bilayer. J. Chem. Phys. 133(15), 154705 (2010)
Gómez, J., Hilser, V.J., Xie, D., Freire, E.: The heat capacity of proteins. Proteins Struct. Funct. Bioinf. 22(4), 404–412 (1995)
Tian, W., Lin, M., Tang, K., Liang, J., Naveed, H.: High-resolution structure prediction of β-barrel membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 115(7), 1511–1516 (2018)
Lervik, A., Bresme, F., Kjelstrup, S., Bedeaux, D., Rubi, J.M.: Heat transfer in protein–water interfaces. Phys. Chem. Chem. Phys. 12(7), 1610–1617 (2010)
Youssefian, S., Rahbar, N., Lambert, C.R., Van Dessel, S.: Variation of thermal conductivity of DPPC lipid bilayer membranes around the phase transition temperature. J. R. Soc. Interface 14(130), 20170127 (2017)
Bastos, A.R.N., Brites, C.D.S., Rojas-Gutierrez, P.A., DeWolf, C., Ferreira, R.A.S., Capobianco, J.A., Carlos, L.D.: Thermal properties of lipid bilayers determined using upconversion nanothermometry. Adv. Func. Mater. 29(48), 1905474 (2019)
Andersen, O.S., Koeppe, R.E.: Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36(1), 107–130 (2007)
Zharov, V.P., Lapotko, D.O.: Photothermal imaging of nanoparticles and cells. IEEE J. Sel. Top. Quantum Electron. 11(4), 733–751 (2005)
Tzou, D.Y.: The generalized lagging response in small-scale and high-rate heating. Int. J. Heat Mass Transf. 38(17), 3231–3240 (1995)
Shomali, Z., Kovács, R., Ván, P., Kudinov, I.V., Ghazanfarian, J.: Lagging heat models in thermodynamics and bioheat transfer: a critical review. Continuum Mech. Thermodyn. 34(3), 637–679 (2022)
Horny, N., Chirtoc, M., Fleming, A., Hamaoui, G., Ban, H.: Kapitza thermal resistance studied by high-frequency photothermal radiometry. Appl. Phys. Lett. 109(3), 033103 (2016)
Zhang, Y.: Generalized dual-phase lag bioheat equations based on nonequilibrium heat transfer in living biological tissues. Int. J. Heat Mass Transf. 52(21), 4829–4834 (2009)
Nakano, T., Kikugawa, G., Ohara, T.: Molecular heat transfer in lipid bilayers with symmetric and asymmetric tail chains. J. Heat Transf. 135(061301), (2013)
Marti, D., Aasbjerg, R.N.N., Andersen, P.E.E., Hansen, A.K.K.: MCmatlab: an open-source, user-friendly, MATLAB-integrated three-dimensional Monte Carlo light transport solver with heat diffusion and tissue damage. J. Biomed. Opt. 23(12), 121622 (2018)
Almeida, P.F., Carter, F.E., Kilgour, K.M., Raymonda, M.H., Tejada, E.: Heat capacity of DPPC/cholesterol mixtures: comparison of single bilayers with multibilayers and simulations. Langmuir 34(33), 9798–9809 (2018)
Blume, A.: Apparent molar heat capacities of phospholipids in aqueous dispersion. Effects of chain length and head group structure. (1983)
Ge, Z., Cahill, D.G., Braun, P.V.: Thermal conductance of hydrophilic and hydrophobic interfaces. Phys. Rev. Lett. 96(18), 186101 (2006)
Nakano, M., Arai, Y., Kotera, I., Okabe, K., Kamei, Y., Nagai, T.: Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS ONE 12(2), e0172344 (2017)
Suzuki, M., Plakhotnik, T.: The challenge of intracellular temperature. Biophys. Rev. 12(2), 593–600 (2020)
Acknowledgements
We thank Prof LD Ziegler, Prof Ji-Xin Cheng, and their groups for discussions and acknowledge the support from the National Institutes of Health (123456 and R01GM142012) and the National Science Foundation (NSF ECCS-1846659).
Funding
Support is acknowledged from the National Institutes of Health (123456 and R01GM142012) and the National Science Foundation (NSF ECCS-1846659).
Author information
Authors and Affiliations
Contributions
PDS carried out the simulations, supervised by MYS and assisted by SE. ON worked out the theory. SE and MKH drafted the manuscript, and all authors contributed to the final writing.
Corresponding authors
Ethics declarations
Informed consent
N/A (no animal or human studies).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samolis, P.D., Sander, M.Y., Hong, M.K. et al. Thermal transport across membranes and the Kapitza length from photothermal microscopy. J Biol Phys 49, 365–381 (2023). https://doi.org/10.1007/s10867-023-09636-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-023-09636-0