Abstract
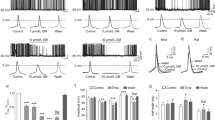
There is an increasing interest in the biological and therapeutic effects of fisetin, a natural phenolic compound. Fisetin has affinity on some neuronal targets and may have the potential to modulate neuronal activity. In this study the effects of acute application of fisetin on synchronized events were evaluated electro-physiologically. Besides, interaction of fisetin with closely related channels were investigated in silico. Acute horizontal hippocampal slices were obtained from 32- to 36-day-old C57BL/6 mice. Extracellular field potentials were recorded from CA3 region of the hippocampus. Bath application of 4 aminopyridine (4AP, 100 µM) initiated ictal- and interictal-like synchronized epileptiform discharges in the brain slices. Fifty micromolar fisetin was applied to the recording chamber during the epileptiform activity. The duration and frequencies of both ictal-like and interictal-like activities were calculated from the electrophysiological records. Molecular docking was performed to reveal interaction of fisetin on GABA-A, NMDA, AMPA receptors, and HCN2 channel, which are neuronal structures directly involved in recorded activity. Although fisetin does not affect basal neuronal activity in brain slice, it reduced the duration of ictal-like discharges significantly. Molecular docking results indicated that fisetin has no effect on GABA-A, NMDA, and AMPA receptors. However, fisetin binds to the (5JON) HCN2 channel strongly with the binding energy of −7.66 kcal/mol. Reduction on the duration of 4AP-induced ictal-like discharges can be explained as HCN channels can cause an inhibitory effect via enhancing M-type K + channels which increase K outward currents.







Similar content being viewed by others
References
Milligan, T.A.: Epilepsy: a clinical overview. Am. J. Med. (2021). https://doi.org/10.1016/j.amjmed.2021.01.038
Sucher, N.J., Carles, M.C.: A pharmacological basis of herbal medicines for epilepsy. Epilepsy Behav. (2015). https://doi.org/10.1016/j.yebeh.2015.05.012
Ravula, A.R., Teegala, S.B., Kalakotla, S., Pasangulapati, J.P., Perumal, V., Boyina, H.K.: Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: an updated review. Eur. J. Pharmacol. (2021). https://doi.org/10.1016/j.ejphar.2021.174492
Grynkiewicz, G., Demchuk, O.M.: New perspectives for fisetin. Front. Chem. (2019). https://doi.org/10.3389/fchem.2019.00697
Maher, P.: Modulation of the neuroprotective and anti-inflammatory activities of the flavonol fisetin by the transition metals iron and copper. Antioxidants (2020). https://doi.org/10.3390/antiox9111113
Imran, M., Saeed, F., Gilani, S.A., Shariati, M.A., Imran, A., Afzaal, M., Atif, M., Tufail, T., Anjum, F.M.: Fisetin: an anticancer perspective. Food Sci. Nutr. (2020). https://doi.org/10.1002/fsn3.1872
Krasieva, T.B., Ehren, J., O’Sullivan, T., Tromberg, B.J., Maher, P.: Cell and brain tissue imaging of the flavonoid fisetin using label-free two-photon microscopy. Neurochem. Int. (2015). https://doi.org/10.1016/j.neuint.2015.08.003
He, W.B., Abe, K., Akaishi, T.: Oral administration of fisetin promotes the induction of hippocampal long-term potentiation in vivo. J. Pharmacol. Sci. (2018). https://doi.org/10.1016/j.jphs.2017.12.008
Cordaro, M., D’Amico, R., Fusco, R., Peritore, A.F., Genovese, T., Interdonato, L., Franco, G., Arangia, A., Gugliandola, E., Crupi, R., Siracusa, R., Di Paola, R., Cuzzocrea, S., Impellizzeri, D.: Discovering the effects of fisetin on nf-κb/nlrp-3/nrf-2 molecular pathways in a mouse model of vascular dementia induced by repeated bilateral carotid occlusion. Biomedicines (2022). https://doi.org/10.3390/biomedicines10061448
Zhen, L., Zhu, J., Zhao, X., Huang, W., An, Y., Li, S., Du, X., Lin, M., Wang, Q., Xu, Y., Pan, J.: The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav. Brain Res. (2012). https://doi.org/10.1016/j.bbr.2011.12.017
Raygude, K.S., Kandhare, A.D., Ghosh, P., Bodhankar, S.L.: Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomed. Prev. Nutr. (2012). https://doi.org/10.1016/j.bionut.2012.04.005
Ramírez, D., Zúñiga, R., Concha, G., Zúñiga, L.: HCN channels: new therapeutic targets for pain treatment. Molecules (2018). https://doi.org/10.3390/molecules23092094
Rivolta, I., Binda, A., Masi, A., DiFrancesco, J.C.: Cardiac and neuronal HCN channelopathies. Pflügers Arch. Eur. J. Physiol. (2020). https://doi.org/10.1007/s00424-020-02384-3
DiFrancesco, J.C., Castellotti, B., Milanesi, R., Ragona, F., Freri, E., Canafoglia, L., Franceschetti, S., Ferrarese, C., Magri, S., Taroni, F., Costa, C., Labate, A., Gambardella, A., Solazzi, R., Binda, A., Rivolta, I., Di Gennaro, G., Casciato, S., Gellera, C.: HCN ion channels and accessory proteins in epilepsy: genetic analysis of a large cohort of patients and review of the literature. Epilepsy Res. (2019). https://doi.org/10.1016/j.eplepsyres.2019.04.004
Carlson, A.E., Rosenbaum, J.C., Brelidze, T.I., Klevit, R.E., Zagotta, W.N.: Flavonoid regulation of HCN2 channels. J. Biol. Chem. (2013). https://doi.org/10.1074/jbc.M113.501759
Sharifi-Rad, J., Quispe, C., Herrera-Bravo, J., Martorell, M., Sharopov, F., Tumer, T.B. Kurt, B., Lankatillake, C., docea, A.O., Moreira, A.C., Dias, D.A., Mahomoodally, M.F., Lobine, D., Cru-Martins, N., Kumar, M., Calina, D.: A pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem. Res. (2021). https://doi.org/10.1007/s11064-021-03376-0
Ozturk, H., Yorulmaz, N., Durgun, M., Basoglu, H.: In silico investigation of Alliin as potential activator for AMPA receptor. Biomed. Phys. Eng. Expr. (2021). https://doi.org/10.1088/2057-1976/ac351c
Aydin-Abidin, S., Abidin, İ: 7,8-Dihydroxyflavone potentiates ongoing epileptiform activity in mice brain slices. Neurosci. Lett. (2019). https://doi.org/10.1016/j.neulet.2019.03.013
Abidin, İ, Aydin-Abidin, S., Mittmann, T.: Neuronal excitability and spontaneous synaptic transmission in the entorhinal cortex of BDNF heterozygous mice. Neurosci. Lett. (2019). https://doi.org/10.1016/j.neulet.2018.10.019
Basoglu, H., Ozturk, H., Keser, H., Aydin-Abidin, S., Abidin, I.: L-Theanine reduces epileptiform activity in brain slices. Akd. Tıp D. (2022). https://doi.org/10.53394/akd.1057342
D’Arcangelo, G., Panuccio, G., Tancredi, V., Avoli, M.: Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol. Dis. (2005). https://doi.org/10.1016/j.nbd.2004.11.012
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., Olson, A.J.: AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. (2009). https://doi.org/10.1002/jcc.21256
Hanwell, M.D., Curtis, D.E., Lonie, D.C., Vandermeersch, T., Zurek, E., Hutchison, G.R.: Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. (2012). https://doi.org/10.1186/1758-2946-4-17
Bechthold, E., Schreiber, J.A., Lehmkuhl, K., Frehland, B., Schepmann, D., Bernal, F.A., Daniliuc, C., Alvarez, I., Garcia, C.V., Schmidt, T.J., Seebohm, G., Wünsch, B.: Ifenprodil stereoisomers: synthesis, absolute configuration, and correlation with biological activity. J. Med. Chem. (2021). https://doi.org/10.1021/acs.jmedchem.0c01912
Rogawski, M.A., Hanada, T.: Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol. Scand. Suppl. (2013). https://doi.org/10.1111/ane.12100
Gong, P., Hong, H., Perkins, E.J.: Ionotropic GABA receptor antagonism-induced adverse outcome pathways for potential neurotoxicity biomarkers. Biomark. Med. (2015). https://doi.org/10.2217/bmm.15.58
Severina, H.I., Georgiyants, V.A., Kovalenko, S.M., Avdeeva, N.V., Yarcev, A.I., Prohoda, S.N.: Molecular docking studies of N-substituted 4-methoxy-6-oxo-1-aryl-pyridazine-3-carboxamide derivatives as potential modulators of glutamate receptors. Res. Results Pharmacol. (2020). https://doi.org/10.3897/rrpharmacology.6.52026
Gallagher, M.J., Huang, H., Pritchett, D.B., Lynch, D.R.: Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. (1996). https://doi.org/10.1074/jbc.271.16.9603
Di Bonaventura, C., Labate, A., Maschio, M., Meletti, S., Russo, E.: AMPA receptors and perampanel behind selected epilepsies: current evidence and future perspectives. Expert Opin. Pharmacother. (2017). https://doi.org/10.1080/14656566.2017.1392509
Sigel, E., Steinmann, M.E.: Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. (2012). https://doi.org/10.1074/jbc.R112.386664
Zhu, S., Noviello, C.M., Teng, J., Walsh, R.M., Jr., Kim, J.J., Hibbs, R.E.: Structure of a human synaptic GABA(A) receptor. Nature (2018). https://doi.org/10.1038/s41586-018-0255-3
Robinson, R.B., Siegelbaum, S.A.: Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. (2003). https://doi.org/10.1146/annurev.physiol.65.092101.142734
Craven, K.B., Zagotta, W.N.: CNG and HCN channels: two peas, one pod. Annu. Rev. Physiol. (2006). https://doi.org/10.1146/annurev.physiol.68.040104.134728
Goldschen-Ohm, M.P., Klenchin, V.A., White, D.S., Cowgill, J.B., Cui, Q., Goldsmith, R.H., Chanda, B.: Structure and dynamics underlying elementary ligand binding events in human pacemaking channels. Struct. Biol. Mol. Biophys. (2016). https://doi.org/10.7554/eLife.20797
Meng, X.Y., Zhang, H.X., Mezei, M., Cui, M.: Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. (2011). https://doi.org/10.2174/157340911795677602
Isika, D., Çeşme, M., Osonga, F.J., Sadik, O.A.: Novel quercetin and apigenin-acetamide derivatives: design, synthesis, characterization, biological evaluation and molecular docking studies. RSC Adv. (2020). https://doi.org/10.1039/D0RA04559D
Bissantz, C., Folkers, G., Rognan, D.: Protein-based virtual screening of chemical databases. 1. Evaluation of different docking/scoring combinations. J. Med. Chem. (2000). https://doi.org/10.1021/jm001044l
Kase, D., Imoto, K.: The role of HCN Channels on membrane excitability in the nervous system. J. Signal Transduct. (2012). https://doi.org/10.1155/2012/619747
George, M.S., Abbott, L.F., Siegelbaum, S.A.: HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat. Neurosci. (2009). https://doi.org/10.1038/nn.2307
Lin, W., Qin, J., Ni, G., Li, Y., Xie, H., Yu, J., Li, H., Sui, L., Guo, Q., Fang, Z., Zhou, L.: Downregulation of hyperpolarization-activated cyclic nucleotide-gated channels (HCN) in the hippocampus of patients with medial temporal lobe epilepsy and hippocampal sclerosis (MTLE-HS). Hippocampus (2020). https://doi.org/10.1002/hipo.23219
Das, J., Singh, R., Sharma, D.: Antiepileptic effect of fisetin in iron-induced experimental model of traumatic epilepsy in rats in the light of electrophysiological, biochemical, and behavioral observations. Nutr. Neurosci. (2017). https://doi.org/10.1080/1028415x.2016.1183342
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozturk, H., Basoglu, H., Yorulmaz, N. et al. Fisetin decreases the duration of ictal-like discharges in mouse hippocampal slices. J Biol Phys 48, 355–368 (2022). https://doi.org/10.1007/s10867-022-09612-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-022-09612-0